



VOL. 32, NO. 1 , Spring 2023 12 The callouts will go below in this space 56 The callouts will go below in this space 23 The callouts will go below in this space 73 The callouts will go below in this space Neuromorphic Computing VOL. 32, NO. 1, Spring 2023 47 Building a Smart and Green AI 14 A Sneak Peek at the 243rd Meeting 49 Emerging Memory Devices: Paving the Path for Energy-Efficient BrainInspired Computing 40 Engineering the Neuronal Response to Electrical Microstimulation
May 28-June 2, 2023
SWEDEN
October 8-12, 2023
Joint International Meeting of ECS, ECSJ, and KECS
October 6-11, 2024
FUTURE ECS MEETINGS www.electrochem.org/meetings EQUATO TROPIC OF CAPRICORN ANTARCTIC CIRCLE ARCTIC CIRCLE
HONOLULU, HI 244 243 245 PRIME 2024
244th ECS Meeting GOTHENBURG 245th ECS Meeting SAN FRANCISCO, CA PRiME 2024 Joint International Meeting
245th ECS Meeting SAN FRANCISCO, CA May 26-30, 2024 Marriot
Marquis San Francisco
244th ECS Meeting
GOTHENBURG
R
Swedish Exhibition &
Congress
Centre TROPIC OF CANCE
PRiME 2024
HONOLULU, HI
Hawaii Convention Center & Hilton Hawaiian Village
243rd ECS Meeting BOSTON, MA
243rd ECS Meeting with SOFC-XVIII BOSTON, MA
Hynes Convention Center and Sheraton Boston


Published by: The Electrochemical Society (ECS) 65 South Main Street Pennington, NJ 08534-2839, USA Tel 609.737.1902, Fax 609.737.2743 www.electrochem.org
Tell Them Now
Iam getting to that point in life when friends start leaving, and I am not a big fan. I just found out that a friend of mine from college died from an aggressive form of amyotrophic lateral sclerosis, better known as ALS. In May he did a 50-mile bike ride with his cycling friends, in August he received the diagnosis, and in December he entered hospice care at age 61. His son’s wedding in the spring will be missing a very important person. I cannot imagine the grief. We had played soccer together in college, and while we were in touch only sporadically over the years, it always felt that we could pick up where we left off. He always had a smile, and he had a way of making those around him just feel better. I didn’t see him between diagnosis and death. Other friends and family have been lost over the years, but this one struck me a lot harder as it drives home the point that all of us have hourglasses for our time on Earth, but none of us know how much sand is in them, and I am certainly at the point where there is more in the bottom of the hourglass than in the top.
On a related but happier note, at the 242nd Meeting in Atlanta last fall, the Corrosion Division of ECS had a symposium in honor of Jerry Frankel of Ohio State. Jerry served as the Corrosion Science and Technology Technical Editor for the Journal of the Electrochemical Society for many years and was one of the original organizers for the “Critical Factors in Localized Corrosion” symposium that has been going on for 20 years, amongst many, many other achievements and contributions. It was three full days of talks, including some from special guests, as well as a rousing reception where his family was able to join by Zoom for the various speeches, including one from his PhD advisor. As a side note, let this be a cautionary tale for current PhD students – you can never escape your advisor; we follow you everywhere. The appreciations and laudatory comments were all heartfelt, but probably most had not been expressed to Jerry before. It wasn’t until the symposium that people (including me) took the time to reflect on what Jerry has meant to them, and then to tell him. Watching Jerry during that time I could see how much it all meant to him, hearing the range and depth of impact he has had on people, not just on science. Retirement parties (not that Jerry’s reception was that though he has a bit more gray hair than when I met him years ago) are another time when we take the opportunity to let others know how appreciated they are and for what.
Both experiences have made me feel that I should be a lot more proactive in telling people how much I value them (of course, I will be nice and restrict it only to the people whom I value positively). That is not as easy as it seems for me, because there is no “right” time. I find it is easier for those who are at a distance; I have two mentors to whom I send appreciations each Father’s Day, their impact on me has been so great. In that medium, I can compose and edit what I want to say, and there is no need for response. If I were to tell people who are physically closer, I would run the risk of developing blurred vision due to something getting in my eye and then blabbing on like an idiot. Some may wonder how blabbing like an idiot would be any different than my normal speaking, but they are just mean. I am working on getting over that hurdle, but good commercials make me cry, so I may just have to live with it. All of us have been blessed with many people in our lives who raise us up when we stumble or inspire us to be better versions of ourselves. It makes little sense not to let them know that while they can appreciate it.
Until next time, be safe and happy.
Editor: Rob Kelly
Guest Editor: Durgamadhab Misra
Contributing Editors: Christopher L. Alexander, Chris Arges, Scott Cushing, Ahmet Kusolgu, Donald Pile, Alice Suroviec
Director of Publications: Adrian Plummer
Director of Community Engagement: Shannon Reed
Production Editor: Kara McArthur
Graphic Design & Print Production Manager: Dinia Agrawala
Staff Contributors: Frances Chaves, Genevieve Goldy, Mary Hojlo, Christopher J. Jannuzzi, John Lewis, Anna Olsen, Jennifer Ortiz, Beth Schademann, Francesca Spagnuolo
Advisory Board: Brett Lucht (Battery), Dev Chidambaram (Corrosion), Durga Misra (Dielectric Science and Technology), Philippe Vereecken (Electrodeposition), Jennifer Hite (Electronics and Photonics), Mani Manivannan (Energy Technology), Cortney Kreller (High-Temperature Energy, Materials, & Processes), John Weidner (Industrial Electrochemistry and Electrochemical Engineering), Jakoah Brgoch (Luminescence and Display Materials), Hiroshi Imahori (Nanocarbons), James Burgess (Organic and Biological Electrochemistry), Robbyn Anand (Physical and Analytical Electrochemistry), Ajit Khosla (Sensor)
Publications Subcommittee Chair: Colm O'Dwyer
Society Officers: Turgut Gür, President; Gerardine Botte, Senior Vice President; Colm O’Dwyer, 2nd Vice President; James (Jim) Fenton, 3rd Vice President; Marca Doeff, Secretary; Elizabeth J. Podlaha-Murphy, Treasurer; Christopher J. Jannuzzi, Executive Director & CEO
Statements and opinions given in The Electrochemical Society Interface are those of the contributors, and ECS assumes no responsibility for them.

Authorization to photocopy any article for internal or personal use beyond the fair use provisions of the Copyright Act of 1976 is granted by The Electrochemical Society to libraries and other users registered with the Copyright Clearance Center (CCC). Copying for other than internal or personal use without express permission of ECS is prohibited. The CCC Code for The Electrochemical Society Interface is 1064-8208/92.
ISSN : Print: 1064-8208 Online: 1944-8783
The Electrochemical Society Interface is published quarterly by The Electrochemical Society (ECS), at 65 South Main Street, Pennington, NJ 08534-2839 USA.

Subscription to members is part of membership service.
© Copyright 2023 by The Electrochemical Society. *“Save as otherwise expressly stated.”
The Electrochemical Society is an educational, nonprofit 501(c)(3) organization with more than 8,500 scientists and engineers in over 75 countries worldwide who hold individual membership. Founded in 1902, the Society has a long tradition in advancing the theory and practice of electrochemical and solid state science by dissemination of information through its publications and international meetings.
Rob Kelly Editor https://orcid.org/0000-0002-7354-0978
FROM THE EDITOR FROM THE EDITOR
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 3
“All of us have been blessed with many people in our lives who raise us up when we stumble or inspire us to be better versions of ourselves. It makes little sense not to let them know that while they can appreciate it.ˮ



4 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org Rapid, High-Current Pulsing & Superior, Low-Impedance EIS www.gamry.com Reference 30K Booster Nobody is faster Distortion-free, rapid pulsing for charge and discharge 30 A Pulse on a 5 mOhm cell Nobody goes lower Up to 30A @ 20V, EIS to 300 kHz 200 nOhm Shorted Lead Curve 20 A rms, 0.9 m cell cable
Vol. 32, No. 1 Spring 2023
37 40 45 47 49
Reports from the Frontier: Heterogeneous Electrocatalysts for Sustainable Electrochemical Synthesis
by Zachary J. Schiffer
edited by Scott Cushing
Electrochemistry in Action: Engineering the Neuronal Response to Electrical Microstimulation
 by M. E. Orazem and Kevin J. Otto
edited by Christopher L. Alexander
by M. E. Orazem and Kevin J. Otto
edited by Christopher L. Alexander
Special Issue of Interface on Neuromorphic Computing: An Introduction and State of the Field
by Durgamadhab Misra, Special Issue Guest Editor
Building a Smart and Green AI
by Bipin Rajendran
Emerging Memory Devices Beyond Conventional Data Storage: Paving the Path for Energy-Efficient Brain-Inspired Computing

 by Rashmi Jha
by Rashmi Jha
3 From the Editor: Tell Them Now
7 Pennington Corner: Driving the Clean Energy Revolution
10
2022 Year in Review
14 243rd ECS Meeting with SOFC-XVIII Boston, MA
20 Society News
26 Podcasts of Note
29 People News
31 Looking at Patent Law
43 Tech Highlights
52 Section News
54 Awards Program
61 New Members
64 Student News
69 Call for Papers
244th ECS Meeting, Gothenburg, Sweden
This
month's cover, designed by Dinia Agrawala, evokes this issue’s theme of brain-inspired computing or neuromorphic computing. These architectures and systems aim to mimic the human brain’s incredibly energy-efficient ability to perform complex cognitive tasks.
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 5

6 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org
Driving the Clean Energy Revolution
One of the most inspiring and rewarding aspects of my role with ECS is the opportunity to support the greatest minds in the electrochemical and solid state sciences as they strive to meet the grand global challenges facing the world today, such as combating climate change, finding renewable energy sources, and providing secure, high-speed connectivity to all. As someone who is neither a scientist nor a researcher, I am very grateful for this immense honor, which fills me with a sense of purpose and pride in my work.
However, a downside of my job is that not a lot of people outside of the science world know that groups like ECS exist and so it is often difficult to explain to people exactly what I do for a living. Fortunately, the increasing integration of science and technology into our daily lives provides the opportunity to share with the general public the critical role technical societies play and why these organizations matter so much.
For example, I recently purchased an electric car (EV). When I contacted my electrician to have a Level 2 EV charger installed at my house, he asked me which type of charger I would like. He added, “You probably want the SAE J1772. It’s the most common, but I have no idea what SAE stands for.”
“SAE stands for the Society for Automotive Engineers,” I responded. “Actually, I have a meeting with them next week to discuss how we can partner to advance EV adoption in the US!” We were both surprised at my enthusiastic response, but this matters a great deal to me.
“For real?” he said.
“Yes,” I answered and proceeded to give my electrician a brief—well, my electrician may not have thought it was brief—history of technical societies and the founding of peer review. I went all the way back to Henry Oldenburg when he was Secretary of the Royal Society in the mid-17th century. Of course, London in the 1650s may seem like a long way off from 21st century suburban New Jersey, US. However, it was not difficult to connect that rich historical legacy to the world where my electrician and I live, in the physical shadow of Bell Labs’ New Jersey headquarters and the incredible technological advancements discovered there.
It was a wonderful moment for me. As I have written in previous articles, I am very grateful for the advancements made by the ECS community. Consider the potential of EVs to address climate change and to make the world a better place. Now consider the number of ECS technologies involved in making this EV revolution possible. Simply put, this change is driven by the technologies in ECS’ fields of interest— from batteries and fuel cells to sensors and photovoltaics, semiconductors and LEDs—and the people working in these technologies.
This is my first EV. I doubt I will ever buy a car with a traditional internal combustion engine (ICE) again. Along with this car’s incredible responsiveness and quiet comfort compared to an ICE, it’s amazing to drive the 125-mile-roundtrip commute between my home and the ECS office with almost no additional environmental impact! Furthermore, not only is this a far greener mode of transportation, it now costs me significantly less to drive to work. I charge my car with the excess solar power generated by my house’s solar. Or, I use any number of DC fast chargers available at no cost through an agreement between my car’s manufacturer and a major US EV charging provider.
That’s real progress, even though we still have a very long way to go until clean renewable energy is as ubiquitous as the gasoline filling stations we want to make obsolete. However, thanks to the ECS community, we are moving quickly in the right direction. To be part of this absolutely vital work is an honor I treasure every day.
Thank you all!
Christopher J. Jannuzzi ECS Executive Director/Chief Executive Officer https://orcid.org/0000-0002-7293-7404


The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 7 FROM THE PRESIDENT FROM THE PRESIDENT
“Fortunately, the increasing integration of science and technology into our daily lives provides the opportunity to share with the general public the critical role technical societies play and why these organizations matter so much.”
VMP-300 16 channels, ± 150 A down to 100 fA
SP-200
1 channel ± 500 mA down to 100 fA
VSP-300

6 channels ± 40 A down to 100 fA

SP-240
1 channel
± 4 A down to 100 fA
SP-300
2 channels ± 10 A down to 100 fA
BioLogic Premium is a range of state-of-the-art potentiostats designed for researchers who need the fastest, most precise, potentiostats available.



Premium potentiostats boast some of the most powerful specifications available: 100 fA to 150 A, 7 MHz EIS measurements and a sampling rate that can reach 1 data point every µs.
Premium by name. Premium by nature.
Shaping the future. Together.
Click or scan
Premium.
When only the best will do
Essential. Tools for Electrochemists

VMP-3e 16 channels, ± 1 A down to 20 nA

SP-150e
2 channels ± 1 A down to 20 nA
VSP-3e 8 channels ± 1 A down to 20 nA
SP-50e 1 channel ± 1 A down to 20 nA
A range of powerful, modular, high-precision, research-grade potentiostats built to handle almost any academic or industrial application imaginable.


From 1 to 16 channels. 1 A (native), up to 800 A with boosters, EIS and Quality Indicators. Essential measurement tools. Whatever your area of specialisation.
Click or scan
www.biologic.net
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 9
2022 Year in Review
While the tumultuous 2020s continue to present the Society and the world with myriad, unprecedented challenges, the ECS community endured these trying times and continued to further the Society’s vital mission of advancing our science for the betterment of all humanity.
In 2022, we:
• Successfully launched two new open access journals, ECS Sensors Plus and ECS Advances; the first issues were distributed in March and April respectively, with more than 17 articles submitted and 3,068 downloaded (despite only opening for submissions in January 2022);
• Returned to in-person meetings; more than 4,000 members and constituents came together at our biannual conferences in Vancouver, Canada (spring), and Atlanta, GA (fall);
• Engaged and championed inclusivity, honoring and supporting members’ accomplishments at every career level, and launched new awards, including the John B. Goodenough Award of The Electrochemical Society, which recognizes distinguished contributions to the fundamental and technological aspects of electrochemical materials science and engineering;
• Expanded the ECS member base; more than 1,000 new members joined the ECS community;
• Exercised disciplined, prudent fiscal stewardship; maintained our strong financial position despite global inflation and a volatile economic climate.
The following report provides greater detail on the Society’s 2022 operations and performance, and on how the members, volunteers, and staff together supported the ECS mission of advancing theory and practice at the forefront of electrochemical and solid state science and technology, and allied subjects.
Total Members: 7,132 Student members: 2,329
MEMBERSHIP Highlights
Overall Membership Retention: 71% (increase from 58% in 2021)
Institutiona l members:
With the return to in-person meetings, membership began rebounding. In 2022, the number of members in good standing (i.e., dues-paying members) increased by 1,040 individual members. In addition, for the
first time, the Society hosted in-person member receptions at both ECS biannual meetings—each event bringing more than 500 members together to network.


10 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org
Members in g ood standing: +20.8% +37.9% S t u dent members in goodstandi n g : 123 Totalstude n t chapters: +10 +7 2021 2021 2022 2022 47
ECS Fellows
FELLOWSHIPS AND AWARDS
ECSSummer Fellowships
ECS Summer Fellowships


Number of Fellows inducted into the 2022 class: 15
Total awarded: $250,000
Total awarded: $20,000
Number of recipients: 4 at $5,000 each
ECS Colin Garfield Fink Summer Fellowship
Travel Grants
241st ECS Meeting
Total awarded: $47,135
Number of recipients: 111
242nd ECS Meeting
ECSToyotaYoung InvestigatorFellowship s
Number of recipients: 5 at $50,000 each
Student
Total awarded: $5,000
Number of recipients: 1
Total awarded: $59,160
Number of recipients: 102
SOCIETY, DIVISION, AND SECTION PRIZES
Poster Awards
Total awarded: $5,000
Number of poster winners: 5
Society,Division, & Section Awards Student
Poster Awards
241st ECS Meeting
1st prize: $1,500 2nd prize: $1,000
242nd ECS Meeting
1st prize: $1,500 2nd prize: $1,000
Highlights
The Honors & Awards Program continues to showcase outstanding technical achievement in electrochemical and solid state science, and to recognize exceptional service to the Society. ECS awarded $28,000 for eight Society awards; $21,500 for 31 division awards; and $8,000 for 11 section awards.
The Society proudly announces the 2022 ECS Class of Fellows: Michel Armand, Perla Balbuena, Gerd Ceder, Wilson Chiu, Andrew Hoff, Gao Liu, Brett Lucht, Janine Mauzeroll, Nguyen Minh, Deborah Myers, James Noël, Elizabeth
Total awarded: $57,500
Total number of recipients: 50 Society: 8 Division: 31 Section: 11
Podlaha-Murphy, Vijay Ramani, Yasuhiro Shimizu, and Shunpei Yamazaki
The new Society award created in honor of longtime ECS member and Nobel laureate John B. Goodenough was a special highlight. ECS President Turgut Gür and Past President Eric Wachsman announced the award at Prof. Goodenough’s 100th Birthday Celebration. He responded, “Thank you all very much, and remember this: one step at a time!”
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 11
MEETINGS
241st ECS Meeting Vancouver, BC, Canada
Participants: 2,174 Symposia: 45
Number of countries represented: 66 Abstracts: 2,515
Exhibitors: 21
Special Events
• 241st ECS Meeting Plenary Speaker: Jeff Dahn (Dalhousie University), “Modern Lithium-Ion Batteries: More than One Million Miles and Possibly a Century of Life”
Participants: 2,614
Symposia: 48
Number of countries represented: 65
Abstracts: 2,634
Exhibitors: 35
Symposium Speaker Funding
Total external symposium funding: $19,875
Total division symposium funding: $26,300
Total registration waivers: $25,282.31
• First time livestreaming Plenary Lecture and award talks
• First time hosting digital presentation files
• Inaugural ECS Member Reception
• Launch of Meet the Editors Program
Symposium Speaker Funding
Total external symposium funding: $160,375
Total division symposium funding: $51,630
Total registration waivers: $71,390
Special Events
• 242nd ECS Meeting Plenary Speaker: M. Stanley Whittingham (Binghamton University), “The Critical Role of Energy Storage in the Electric Economy and Overcoming Climate Change”
• Celebration of 100 Years of the Electrodeposition Division: Past, Present, and Future
• Fulbright Program – Meet the Alumni
Atlanta, GA
• Symposia honoring George Blomgren, Gerald Frankel, D. Noel Buckley, Robert Savinell, George Blasse, JeanMichel Savéant, and Friedrich B. Prinz
ECS Sponsored Meetings
• StorageX International Symposium Series

• Telluride Innovation Workshop: Decarbonization of Cement
• 7th Annual Next Generation Electrochemistry (NGenE) Workshop: Electrochemistry for Decarbonization

• Aqueous Corrosion Gordon Research Conference
• 2022 Workshop on Electrochemical Measurements
• Electrochemistry Gordon Research Seminar
• Electrochemistry Gordon Research Conference
• XXXVII Congreso Nacional de la Sociedad Mexicana de Electroquímica
• Organic Battery Days 2022
12 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org
242nd ECS Meeting
Articles and abstracts downloaded from the ECS Digital Library: 7,934,936

Journal articles published in the 2022 volume year: 2,084
Percentage of articles published open access in 2022: 41%
Open access papers published: 607
Highlights
• Two new journals, ECS Sensors Plus and ECS Advances

• Publications webinar series
• Increase in impact factor for JES and JSS
• Focus Issue In Honor of John Goodenough: A Centenarian Milestone
• Focus Issue on Women in Electrochemistry
• Amost 8 million content downloads
• New Transformative Agreements with more than 200 institutions worldwide to support more open access content for researchers
CONTINUING EDUCATION
Highlights
Webinars: 16
Participants: 6,000+
Speakers: 22
Short Courses: 4
Total registrants: 76
ECS launched a request for proposals in late 2022 to solicit the development of battery workforce development courses. The first round of courses is targeted at MS/PhD audiences to help retrain and/or develop new skills to meet the changes in the battery workforce.
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 13
PUBLICATIONS
0% 5% 10% 15% 20% 25% 30% 0 20 40 60 80 100 120 140 160 180 USA GERMANY JAPAN CANADA CHINA UK INDIA FRANCE KOREA (Republic of) TAIWAN SWITZERLAND SPAIN SWEDEN IRELAND BELGIUM ISRAEL NETHERLANDS AUSTRALIA DENMARK ITALY SLOVENIA THAILAND BRAZIL ESTONIA LITHUANIA MEXICO ROMANIA SOUTH AFRICA AUSTRIA INDONESIA NORWAY RUSSIAN FEDERATION VIET NAM ALGERIA ARGENTINA CHILE FINLAND GREECE HUNGARY KUWAIT LEBANON MALAYSIA PAKISTAN PERU POLAND SAUDI ARABIA SERBIA UKRAINE Total papers published Open Access Percentage of papers from each country
243rd ECS Meeting with SOFC-XVIII
BOSTON, MA, USA l May 28–June 2, 2023
TThe 243rd ECS Meeting and the 18th International Symposium on Solid Oxide Fuel Cells (SOFC-XVIII) both take place in Boston from May 28 to June 2, 2023, at the Hynes Convention Center, Sheraton Boston, and Hilton Boston Back Bay.

Join us at these international conferences as scientists, engineers, and researchers from academia, industry, and government laboratories come together to share results and discuss issues on related topics. Formats include oral presentations, poster sessions, panel discussions, tutorial sessions, short courses, professional development workshops, exhibits, and more! The meeting’s unique blend of electrochemical and solid state science and technology provides the opportunity to absorb and exchange information on the latest scientific developments across a variety of interdisciplinary areas in a forum of your peers.
We are pleased to be joined by SOFC-XVIII, which will feature almost 400 technical presentations over five days from the world’s preeminent researchers on solid oxide fuel cells (SOFCs), solid oxide electrolysis cells (SOECs), and related topics. Please note that a separate registration is required to enjoy the full SOFC meeting, which includes a ticket to the SOFC banquet and a copy of the SOFCXVIII proceedings published in ECS Transactions
This year’s spring meeting is in Boston’s Back Bay, one of the most vibrant and centrally located areas of the city. Be sure to explore the food, shopping, and energy of the nearby Copley Square, South End, Kenmore Square, and Beacon Hill neighborhoods. Take time to enjoy spring blossoms with walks through the Boston Public Gardens, Boston Commons, Charles River Esplanade, or Back Bay Fens. Indulge your inner tourist by walking the Freedom or Black Heritage Trails, taking a duck boat or trolley tour, visiting Harvard Square, or catching a baseball game at Fenway Park!
Start planning now to experience the following technical and networking opportunities:
• Six days of technical programming across 46 symposia
• Over 2,700 abstracts, including almost 400 for SOFC-XVIII
• More than 2,300 oral presentations, including almost 600 invited talks from the world’s leading experts
• Over 350 posters during three evenings of poster sessions
• 14 hours of exhibit hall time over three days
• Daily morning and afternoon coffee breaks
• Complimentary WiFi in meeting rooms
• Special program for nontechnical registrants
AWARD-WINNING SPEAKERS
(Check the Online Program for times)
Society Award–Winning Speakers
Joseph Hupp, Northwestern University
Allen J. Bard Award in Electrochemical Science
Fred Roozeboom, Universiteit Twente, Netherlands
Gordon E. Moore Medal for Outstanding Achievement in Solid State Science and Technology
Arumugam Manthiram, University of Texas at Austin
John B. Goodenough Award of The Electrochemical Society
ECS Division Award–Winning Speakers
Chennupati Jagadish, Australian National University
Dielectric Science and Technology Division Thomas D. Callinan Award
Jean-Michel Hartmann, CEA-Leti Electronics and Photonics Division Award
Adam Weber, Lawrence Berkeley National Laboratory Energy Technology Division Research Award
Yirui Zhang, Massachusetts Institute of Technology
Energy Technology Division Graduate Student Award sponsored by BioLogic
Kelsey Stoerzinger, Oregon State University Energy Technology Division Supramaniam Srinivasan Young Investigator Award
Tatsuya Kawada, Tohoku University
High-Temperature Energy, Materials, & Processes Division Subhash Singhal Award
Bairav Sabarish Vishnugopi, Purdue University
Industrial Electrochemistry and Electrochemical Engineering
Division H. H. Dow Memorial Student Achievement Award
Lauren Clark, Massachusetts Institute of Technology
Industrial Electrochemistry and Electrochemical Engineering Division Student Achievement Award
Francis D’Souza, University of North Texas Nanocarbons Division Robert C. Haddon Research Award
Keith Stevenson, Skolkovo Institute of Science & Technology
Physical and Analytical Electrochemistry Division David C. Grahame Award
SHORT COURSES
Sunday, May 28
ECS short courses are all-day classes designed to provide students and seasoned professionals with in-depth education on a wide range of topics. Taught by academic and industry experts, the small classes provide personalized instruction and help novices and experts advance their technical expertise and knowledge.
Basic Impedance Spectroscopy
Mark Orazem, Instructor
Fundamentals of Electrochemistry: Basic Theory and Thermodynamic Methods
James Noël, Instructor
Lithium-Ion Battery Safety and Failure Modes
Thomas Barrera, Instructor
Electrochemical Capacitor Technology
John Miller, Instructor
14 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 243RD ECS MEETING with SOFC-XVIII • BOSTON, MA • May 28–June 2, 2023
PROFESSIONAL DEVELOPMENT WORKSHOPS
(Check the Online Program for times)
At its biannual meetings, ECS provides professional development programs, such as workshops and professional panels, as well as career resources. ECS’s workshop opportunities are designed for students, early career researchers, and experienced professionals.
ECS Mentoring Session
Facilitator: Alice Suroviec, Berry College
Joint Center for Energy Storage Research (JCESR) Workforce
Development Panel
Instructors: Fikile Brushett, Massachusetts Institute of Technology, and Lynn Trahey, Argonne National Laboratory
Managing and Leading Teams
Instructor: Dennis Hess, Georgia Institute of Technology
Peer Reviewer Excellence Certification Workshop
Instructors: Paul Cooper, The Electrochemical Society, and Jessica MacDonald, IOP Publishing
Win Funding: How to Write a Competitive Proposal
Instructor: Michel Fouré, Berkeley Grant Writing, LLC
SPECIAL EVENTS
(Check the Online Program for times)
Opening Reception
Join us for a taste for Boston and kick off an exciting week! All attendees are welcome for light snacks, open bar, ample time to network, and a chance to meet with ECS divisions.
ECS Members Reception*
ECS members are invited to kick off the meeting with members of the ECS community and food, drinks, giveaways, and light entertainment before the opening reception. Only 400 tickets available!
Student Mixer*
Wrap up the meeting’s first full day with friends and peers at the Student Mixer sponsored by Pine Research. Students and early-career professionals mingle in a relaxed setting and enjoy light hors d’oeuvres and refreshments. Don’t miss it!

Annual Society Business Meeting and Luncheon*
Join us as we celebrate the many successes of 2022 and look forward to an even brighter future!
SOFC Plenary Session
This session opens the SOFC meeting with fuel cell and hydrogen program updates from the US Department of Energy (DOE), Japan’s New Energy and Industrial Technology Development Organization (NEDO), the Korean Institute of Energy Research (KIER), and Europe’s Clean Hydrogen Partnership.
SOFC Banquet*
Take a break from the technical formalities of the SOFC meeting to network and socialize with your peers at this exquisite event, with a reception followed by a formal dinner with entertainment.
General and Student Poster Sessions
With hundreds of posters to explore, you will not want to miss a minute of these sessions. Grab a snack, wander the aisles, review the presentations, talk to the authors, and get to know our exhibitors. These sessions are a great way to end the day!

Technical Exhibition
Take time to explore exhibits from the leading vendors in the electrochemical and solid state science fields. Make sure to stop by the exhibit hall for poster sessions, Networking Breaks, professional portraits, and the ECS Booth.

Division and Symposia Social Events

Plenty of ECS divisions and individual symposia host social events (receptions, banquets, luncheons, and more!) throughout the week. Check the online program for all these opportunities to socialize and network with your peers!
*These events require either pre-registration or purchase of a separate ticket. (continued on next page) www.electrochem.org/24

The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 15 243RD ECS MEETING with SOFC-XVIII • BOSTON, MA • May 28–June 2, 2023
3
(continued from previous page)
Symposium Topics
SOFC— Solid Oxide Fuel Cells
SOFC—Eighteenth International Symposium on Solid Oxide Fuel Cells (SOFC-XVIII)
Eric. D. Wachsman, Teruhisa Horita
High-Temperature Energy, Materials, and Processes Division, SOFC Society of Japan
A Batteries and Energy Storage
A01 New Approaches and Advances in Electrochemical Energy Systems
Ayyakkannu Manivannan, S. R. Narayan, Brett L. Lucht, Loraine
Torres-Castro
Energy Technology Division; Battery Division
A02
A03
Lithium-Ion Batteries
Zheng Li, Ethan Self, Chunsheng Wang
Battery Division
Large Scale Energy Storage 14
Mahendra Sunkara, Joshua Gallaway, Ertan Agar, Christopher G. Arges, D. Noel Buckley
Energy Technology Division; Battery Division; Physical and Analytical Electrochemistry Division
A04 Battery Student Slam 7
Betar Gallant, Lin Ma
Battery Division
A05 Sodium and Zinc Batteries
Xiaolin Li, Feng Lin, Montserrat Galceran, Rachel Carter, Dominic Bresser, Guiliang Xu, Jinchao Huang
Battery Division
A06 Solid State Batteries
Haegyum Kim, Jennifer Schaefer, Ruhul Amin, John Muldoon, Vito
Di Noto
Battery Division
B Carbon Nanostructures and Devices
B01 Carbon Nanostructures for Energy Conversion and Storage
Jeffrey Blackburn, Min-Kyu Song, Xiulei Ji, Andrew Ferguson
Nanocarbons Division
B02 Carbon Nanostructures in Medicine and Biology
Daniel Heller, Ardemis Boghossian, Tatiana DaRos, Markita Landry, Larry Nagahara, Jeffrey Halpern, Mekki Bayachou, Jessica Koehne, Anton Naumov, Nicole Iverson, Noe Alvarez, Delphine
Bouilly
Nanocarbons Division, Sensor Division
B03 Carbon Nanotubes - From Fundamentals to Devices
Ming Zheng, R. Bruce Weisman, Slava V. Rotkin, Shigeo Maruyama, Yan Li, Benjamin Flavel, Yutaka Ohno
Nanocarbons Division
B04 NANO in Spain
Nazario Martín, Tomás Torres, Fernando Langa, Ángela SastreSantos, Hiroshi Imahori
Nanocarbons Division
B05 Fullerenes - Endohedral Fullerenes and Molecular Carbon
Yoko Yamakoshi, Alan Balch, Francis D’Souza, Luis Echegoyen, Dirk Guldi, Nazario Martín, Steven Stevenson, Shangfeng Yang, Akimitsu
Narita
Nanocarbons Division
B06 2D Layered Materials from Fundamental Science to Applications
Michael Scott Arnold, Yaw Obeng, Stefan De Gendt, Zia Karim, Richard Martel, Slava V. Rotkin, Elisa M. Miller-Link
Nanocarbons Division, Dielectric Science and Technology Division
B07 Light Energy Conversion with Metal Halide Perovskites, Semiconductor and Nanostructures, Inorganic/Organic Hybrid Materials, and Dynamic Exciton
Hiroshi Imahori, Prashant Kamat, Kei Murakoshi, Tsukasa Torimoto, Mahesh Hariharan, Zhiqun Lin
Nanocarbons Division
B08 Porphyrins, Phthalocyanines, and Supramolecular Assemblies
Roberto Paolesse, Karl Kadish, Tomás Torres, Nathalie Solladie, Norbert Jux, Ángela Sastre-Santos
Nanocarbons Division
B09 Nano for Industry
Slava Rotkin, Dan Wang, Thorsten Lill, Oana Leonte, David Estrada, Jeff L. Blackburn, Daniel Heller
Nanocarbons Division; Dielectric Science and Technology Division; Industrial Electrochemistry and Electrochemical Engineering
Division; Sensor Division, Interdisciplinary Science and Technology
Subcommittee
C Corrosion Science and Technology
C01 Corrosion General Session
Jamie Noël, Dev Chidambaram
Corrosion Division
D Dielectric Science and Materials
D01 Plasma Electrochemistry and Catalysis 2
Uroš Cvelbar, Mohan R. Sankaran, Davide Mariotti, Mahendra Sunkara
Dielectric Science and Technology Division; Energy Technology

Division
E Electrochemical/Electroless Deposition
E01 Molten Salts (High Temperature) Deposition and Extraction of Metals
Antoine Allanore, Rohan Akolkar, Toshiyuki Nohira, Geir Martin Haarberg, Hojong Kim
Electrodeposition Division; High-Temperature Energy, Materials, and Processes Division
E02 Electrodeposition for Advanced Manufacturing
Timothy Hall, Sudipta Roy, Juan A. Lopez-Ruiz, Massimo Innocenti Electrodeposition Division; Industrial Electrochemistry and Electrochemical Engineering Division
F Electrochemical Engineering
F01 Advances in Industrial Electrochemistry and Electrochemical Engineering: Celebrating 80 Years of the Division
Maria Inman, Paul Kenis, Elizabeth Biddinger, Saket Bhargava Industrial Electrochemistry and Electrochemical Engineering Division
F02 Multiscale Modeling, Simulation, and Design 5: In Honor of Ralph White
Venkat R. Subramanian, Taylor Reed Garrick, John Staser, John Harb, Egwu Eric Kalu, Niloofar Kamyab, Gautam Pillay Industrial Electrochemistry and Electrochemical Engineering Division; Battery Division; Energy Technology Division
F04 Reduction of CO2: From Laboratory to Industrial Scale 3
Christopher Arges, Huyen Dinh, Gang Wu, Plamen B. Atanassov, Saket Bhargava
Industrial Electrochemistry and Electrochemical Engineering Division; Energy Technology Division; Physical and Analytical Electrochemistry Division
F05 Electrochemical Science and Engineering on the Path from Discovery to Product 3
Xiao Su, E. J. Taylor, Karel Bouzek, Saket Bhargava
Industrial Electrochemistry and Electrochemical Engineering Division
16 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 243RD ECS MEETING with SOFC-XVIII • BOSTON, MA • May 28–June 2, 2023
G Electronic Materials and Processing
G01 Silicon Compatible Emerging Materials, Processes, and Technologies for Advanced CMOS and Post-CMOS Applications 13
Hemanth Jagannathan, Zia Karim, Kuniyuki Kakushima, Paul J. Timans, Evgeni Gousev, Stefan De Gendt, Durgamadhab Misra, Yaw Obeng, Fred Roozeboom
Electronics and Photonics Division; Dielectric Science and Technology Division
G02 Processes at the Semiconductor Solution Interface 10 Vidhya Chakrapani, Colm O’Dwyer, D. Noel Buckley, Arnaud Etcheberry, Andrew C. Hillier, Robert Lynch, Philippe Vereecken, Heli Wang, Gautam Banerjee, Sonia Calero-Barney
Electronics and Photonics Division; Dielectric Science and Technology Division; Electrodeposition Division; Physical and Analytical Electrochemistry Division
H Electronic and Photonic Devices and Systems
H01 Wide-Bandgap Semiconductor Materials and Devices
24
Marko Tadjer, Vidhya Chakrapani, Jennifer Hite, Travis Anderson, Steve Kilgore, John Zavada
Electronics and Photonics Division; Dielectric Science and Technology Division
H02 Advanced CMOS-Compatible Semiconductor Devices 20 João Antonio Martino, Bich-Yen Nguyen, Jean-Pierre Raskin, Francisco Gamiz, Siegfried Selberherr, Eddy Simoen, Hiromu Ishii
Electronics and Photonics Division
H03 Solid-State Electronics and Photonics in Biology and Medicine 9
Zong-Hong Lin, Yu-Lin Wang, Wenzhuo Wu, Chih-Ting Lin, Toshiya Sakata, Mark Ming-Cheng Cheng, Lluis Marsal, Shelley Minteer
Electronics and Photonics Division; Physical and Analytical Electrochemistry Division
I Fuel Cells, Electrolyzers, and Energy Conversion
I01 Low Temperature Water Electrolysis (LT-WE) for H2 Production
Hui Xu, Karen E. Swider-Lyons, William Mustain, Marcelo Carmo, Ping Liu, Svitlana Pylypenko, Jingyi Chen Energy Technology Division; Industrial Electrochemistry and Electrochemical Engineering Division; Physical and Analytical Electrochemistry Division
I02 Renewable Fuels via Artificial Photosynthesis or Heterocatalysis 9
Nianqiang Nick Wu, Vaidyanathan Subramanian, Ayyakkannu Manivannan, Pawel J. Kulesza, Frank Osterloh, Bunsho Ohtani, Eric Miller, Gary Wiederrecht, Tianquan Lian, Heli Wang Energy Technology Division
I03 Materials for Low Temperature Electrochemical Systems 9
Minhua Shao, Gang Wu Energy Technology Division
I04 Energy Conversion Based on N, P, and Other Nutrients 2
Lea Winter, Marta Hatzell, William Tarpeh, Gang Wu, Julie Renner Energy Technology Division; Industrial Electrochemistry and Electrochemical Engineering Division
K Organic and Bioelectrochemistry
K01 Organic and Biological Electroanalytical Chemistry: In Memory of Petr Zuman
Sadagopan Krishnan, Jiri Ludvik, James Rusling Organic and Biological Electrochemistry Division
K03 Biomolecular Engineering of Electrochemical Phenomena
Julie Renner, Ariel L. Furst, Jeffrey Halpern, Plamen B. Atanassov Organic and Biological Electrochemistry Division; Energy Technology Division; Physical and Analytical Electrochemistry Division
L Physical and Analytical Electrochemistry, Electrocatalysis, and Photoelectrochemistry
L01 Physical and Analytical Electrochemistry, Electrocatalysis, and Photoelectrochemistry General Session and Grahame Award Symposium
Andrew C. Hillier, Stephen Paddison
Physical and Analytical Electrochemistry Division
L02 Computational Electrochemistry 8
Stephen Paddison, Scott Calabrese Barton, Steven C. DeCaluwe, Shrihari Sankarasubramanian
Physical and Analytical Electrochemistry Division; Energy Technology Division; Industrial Electrochemistry and Electrochemical Engineering Division
L03 Spectroelectrochemistry 6
Andrew C. Hillier, Burcu Gurkan, Yingjie Zhang
Physical and Analytical Electrochemistry Division
L05 Electrochemical Studies by Synchrotron Techniques 2
Anne Co, Svitlana Pylypenko, Kelsey A. Stoerzinger, Iryna Zenyuk
Physical and Analytical Electrochemistry Division; Energy Technology Division
L06 Advances in Analytical Electrochemistry: A Joint Symposium with The Society for Electroanalytical Chemistry (SEAC)
David Cliffel, Alanah Fitch, Bo Zhang
Physical and Analytical Electrochemistry Division; The Society for Electroanalytical Chemistry (SEAC)
L07 Electrochemistry in Extreme Conditions
Pawel J. Kulesza, Vito Di Noto, Iwona Rutkowska, Plamen B. Atanassov, Shrihari Sankarasubramanian
Physical and Analytical Electrochemistry Division; Energy Technology Division; Industrial Electrochemistry and Electrochemical Engineering Division
L09 Fundamental Kinetics and Mechanisms in Environmental and Energy Relevant Reactions
Johna Leddy, David Cliffel, Kelsey A. Stoerzinger, Alanah Fitch
Physical and Analytical Electrochemistry Division; Energy Technology Division
M Sensors
M01 Micro and Nano Systems: In Honor of Peter J. Hesketh
Milad Navaei, Ajit Khosla, Praveen Kumar Sekhar, Gary W. Hunter, Larry A. Nagahara, Thomas G. Thundat, Lok-kun Tsui Sensor Division
M02 Microfluidic Sensors and Devices 4
Jessica Koehne, Nianqiang Wu, Leyla Soleymani, Aida Ebrahimi Sensor Division
Z General
Z01 General General Student Poster Session
Alice Suroviec
All Divisions
Z02 Electrochemical/Materials Processing for Space Engineering
Yasuhiro Fukunaka, Gregory Jackson, George Nelson, Santosh
Vijapur, Antoine Allanore, Donald Sadoway, Thomas Fuller, Ying
Shirley Meng, Robert Kostecki, Vadim F. Lvovich, Bugga
Ratnakumar, Robert Hyers
Electrodeposition Division; Battery Division; Electronics and Photonics Division; Energy Technology Division; HighTemperature Energy, Materials, and Processes Division; Industrial Electrochemistry and Electrochemical Engineering Division; Physical and Analytical Electrochemistry Division; Sensor Division; The Light Metals and the Materials Processing & Manufacturing Divisions of The Minerals, Metals & Materials Society (TMS) www.electrochem.org/24
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 17 243RD ECS MEETING with SOFC-XVIII • BOSTON, MA • May 28–June 2, 2023
3
We are Family!
Journal of The Electrochemical Society
JES is the flagship journal of The Electrochemical Society. Published continuously from 1902 to the present, JES remains one of the most highly cited journals in electrochemistry and solid state science and technology.
ECS Journal of Solid State Science and Technology
JSS is a peer-reviewed journal covering fundamental and applied areas of solid state science and technology, including experimental and theoretical aspects of the chemistry and physics of materials and devices.
18 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org
TODAY! SUBMIT TODAY! Visit Visit www.ecsdl.org www.electrochem.org/focusissues Review the amazing research published by ECS • Calls for upcoming focus issues • Links to published focus issues • Future focus issue proposals
SUBMIT
ECS Sensors Plus
ECS Sensors Plus is a one-stop shop journal for sensors. This multidisciplinary, Gold Open Access journal provides an international platform for publishing high-quality impactful articles and promoting scholarly communication and interactions among scientists, engineers, and technologists whose primary interests focus on materials, structures, properties, performance, and characterization of sensing and detection devices and systems, including sensor arrays and networks.
SUBMIT TODAY!
ECS Advances
ECS Advances is a multidisciplinary, Gold Open Access forum of peer-reviewed, high-quality content covering all technical areas supported by the Society. ECS Advances publishes full-length original work, brief communicationstyle papers, perspectives, review articles, and special issues.
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 19
Visit www.electrochem.org/oa Learn more about our open access initiatives • Read articles published for free in 2023 in ECS Advances and ECS Sensors Plus • Our new Gold Open Access journals!
SUBMIT TODAY!
Publications Update
Realizing Open Access
by Adrian Plummer, MPA, PMP | Director of Publications
A new year has arrived, and ECS and our partner IOP are charting forward into the future like never before. For our volunteers and members, it is no secret that both ECS and IOP firmly believe that the future of science is open, and the recent data on ECS content shows that this is not so much the future as it is the present. In 2022 our hybrid journals, Journal of The Electrochemical Society (JES) and ECS Journal of Solid State Science and Technology (JSS), which offer both subscription and open access content, celebrated a combined 6,250,516 article downloads, a 20% increase from 2021. But the more interesting statistic is that more than 55% of these downloads were of content published using an open access model.
In a piece recently published in Research Information Year Book 2022/2023, in an article titled “Navigating the Shifting Landscape of Scholarly Publishing,” Annabel Ola emphasizes how through open access models researchers have greater access to peer reviewed materials and can now disseminate their work more widely than ever before. Open access was a revolution in scholarly publishing, making research more widely accessible than ever before, but it has also presented some challenges that must be addressed. One of the most cited challenges is the barrier that article processing charges (APCs) create for researchers who do not have the funding for them, either personally or via their institutions. In fact, an international
study by our partner IOP uncovered that 52% of researchers in North America cite lack of funding as a barrier to publishing their content via open access.
In 2023, institutions all over the world started the year with transformative agreements going into effect that will allow authors and researchers to access and publish scientific content in ECS journals without any cost to the author. These institutions include but are not limited to the National Autonomous University of Mexico (UNAM), Statewide California Electronic Library Consortium (SCELC), Council of Australian University Librarians (CAUL), Israeli Inter University Centre for Digital Information Services (MALMAD), Princeton University, the University of Central Florida, Connecticut College, Big Ten Academic Alliance (BTAA), and countless others. These read-and-publish transformative agreements create access for researchers at these institutions all over the globe, to not only read content published behind a paywall, and to publish their work open access fee-free, but they also give energy to scientific advancement, and increase ECS’s standing in the publishing community.
It is embedded in the vision of The Electrochemical Society to facilitate the uninhibited availability of science through open access as a means to accelerate scientific discovery and innovation; thus we must advocate for pathways which remove barriers to the advancement of science.
As you draft your next research article and prepare it for submission, if you would like to publish open access, but are concerned about the potential cost, check the IOP Publishing Journal Finder; you might be surprised at the options available to you.
To learn more about the importance of transformative agreements and the role they play in open access, please be sure to read the article “Transformative Agreements: Making Universal Access to Research a Reality” by our IOP colleague Julian Wilson.
NEXT ISSUE OF IN THE
The Summer 2023 issue of Interface will be guest edited by the Industrial Electrochemistry and Electrochemical Engineering (IE&EE) Division. Summer 2023 will also include results of the 2023 officer elections, columns
from our contributing editors, Looking at Patent Law, Tech Highlights, and the latest news about people, students, and the Society.
The summer issue is scheduled to hit your inbox on June 30!

20 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org SOCIETY NEWS SOCIETY NEWS NEW
Editorial Updates
The Electrochemical Society Publications Subcommittee, Technical Affairs Committee, and Board of Directors congratulate the newly appointed and reappointed members of our Editorial Board. It is through the unwavering commitment of our Editorial Board Members that The Electrochemical Society family of journals continues to realize great success. Thank you for your service to ECS!
Journal of The Electrochemical Society
Perla Balbuena, Associate Editor for Batteries and Energy Storage

April 1, 2021–March 31, 2026
Rajeev Gupta, Associate Editor for Corrosion

January 1, 2021–December 31, 2025
Alice Suroviec, Associate Editor for Physical and Analytical Electrochemistry, Electrocatalysis, and Photoelectrochemistry

February 1, 2017–January 31, 2026
ECS Journal of Solid State Science and Technology
Paul Maggard, Associate Editor for Dielectric Science and Materials

February 19, 2021–February 18, 2026
Meng Tao, Associate Editor for Electronic and Photonic Devices and Systems


May 1, 2019–April 30, 2026
ECS Sensors Plus
Sheng-Joue Young, Associate Editor for Sensors
December 8, 2021–December 8, 2023
Investigate liquid and solid state electrolytes in the new PAT-Cell-Force battery test cell!
The PAT-Cell-Force is a new special operando test cell to adjust and measure the mechanical force applied to the cell stack.
Cell Features:
PAT series test cell for force adjustment and measurement, up to 1500 Newton (up to 5.9 MPa at 18 mm electrode diameter)
For aprotic chemistries with liquid and solid state electrolytes
Built-in gas pressure and temperature sensors

Cableless cell connection via PAT socket, with electronic cell tag
Advanced sealing concept for stable long-term measurements
SOCIETY NEWS SOCIETY NEWS
sales@el-cell.com +49 40 79012-734 el-cell.com


ECS Division Contacts

Battery
Brett Lucht, Chair
University of Rhode Island
Jie Xiao, Vice Chair
Jagjit Nanda, Secretary
Xiaolin Li, Treasurer
Doron Aurbach, Journals Editorial Board Representative
Corrosion
Dev Chidambaram, Chair
University of Nevada Reno
Eiji Tada, Vice Chair
Rebecca Schaller, Secretary/Treasurer
Gerald Frankel, Journals Editorial Board Representative
Dielectric Science and Technology
Uroš Cvelbar, Chair

Jožef Stefan Institute
Sreeram Vaddiraju, Vice Chair
Zhi David Chen, Secretary
Thorsten Lill, Treasurer
Peter Mascher, Journals Editorial Board Representative
Electrodeposition
Natasa Vasiljevic, Chair
University of Bristol
Luca Magagnin, Vice Chair
Andreas Bund, Secretary
Antoine Allanore, Treasurer
Takayuki Homma, Journals Editorial Board Representative
Electronics and Photonics
Qiliang Li, Acting Chair/Vice Chair
George Mason University
Vidhya Chakrapani, 2nd Vice Chair
Zia Karim, Secretary
Erica Douglas, Treasurer
Fan Ren, Journals Editorial Board Representative
Jennifer Bardwell, Journals Editorial Board Representative
Energy Technology

William Mustain, Chair
University of South Carolina
Katherine Ayers, Vice Chair
Minhua Shao, Secretary
Hui Xu, Treasurer
Xiao-Dong Zhou, Journals Editorial Board Representative
High-Temperature Energy, Materials, and Processes

Sean R. Bishop, Chair
Sandia National Laboratories
Cortney Kreller, Senior Vice Chair
Xingbo Liu, Junior Vice Chair
Teruhisa Horita, Secretary/Treasurer
Xiao-Dong Zhou, Journals Editorial Board Representative
Industrial Electrochemistry and Electrochemical Engineering
Maria Inman, Chair
Faraday Technology, Inc.






Paul Kenis, Vice Chair
Elizabeth Biddinger, Secretary/Treasurer
John Harb, Journals Editorial Board Representative
Luminescence and Display Materials

Rong-Jun Xie, Chair
Xiamen University
Eugeniusz Zych, Vice Chair
Dirk Poelman, Secretary/Treasurer
Kailash Mishra, Journals Editorial Board Representative
Nanocarbons


Jeff L. Blackburn, Chair National Renewal Energy Laboratory
Ardemis Boghossian, Vice Chair
Yan Li, Secretary
Hiroshi Imahori, Treasurer
Francis D’Souza, Journals Editorial Board Representative
Organic and Biological Electrochemistry
Sadagopan Krishnan, Chair
Oklahoma State University
Song Lin, Vice Chair
Jeffrey Halpern, Secretary/Treasurer
Janine Mauzeroll, Journals Editorial Board Representative
Physical and Analytical Electrochemistry
Andrew Hillier, Chair Iowa State University
Stephen Paddison, Vice Chair Anne Co, Secretary
Svitlana Pylypenko, Treasurer
David Cliffel, Journals Editorial Board Representative
Sensor
Larry Nagahara, Chair
Johns Hopkins University
Praveen Kumar Sekhar, Vice Chair
Dong-Joo Kim, Secretary
Leyla Soleymani, Treasurer
Ajit Khosla, Journals Editorial Board Representative
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 23
SOCIETY NEWS SOCIETY NEWS
New Division Officer Slates
New officers have been nominated by these ECS divisions for the spring 2023 to spring 2025 term. Election results will be reported in the summer 2023 issue of Interface
Paul Kenis, University of Illinois
Electronics and Photonics Division

Chair
Qiliang Li, George Mason University
Vice Chair
Vidhya Chakrapani, Rensselaer Polytechnic Institute 2nd Vice Chair
Zia Karim, Yield Engineering Systems
Secretary
Helmut Baumgart, Old Dominion University
Treasurer
Erica Douglas, Sandia National Laboratories
Member at Large
Travis J. Anderson, US Naval Research Laboratory
D. Noel Buckley, University of Limerick
Yu Cao, Fast Power, Inc.
Yu Lun Chueh, National Tsing Hua University
Stefan De Gendt, IMEC
M. Jamal Deen, McMaster University
Jennifer Hite, US Naval Research Laboratory
Andrew M. Hoff, University of South Florida
Hiroshi Iwai, National Yang Ming Chiao Tung University
Hemanth Jagannathan, IBM Corporation Research Center
Soohwan Jang, Dankook University
Daisuko Kiriya, The University of Tokyo
Yue Kuo, Texas A&M University
Qizhi Liu, GlobalFoundries, Inc.
Robert Lynch, University of Limerick
Junichi Murota, Tohoku University
Colm O’Dwyer, University College Cork
Takahito Ono, Tohoku University
Mark E. Overberg, Sandia National Laboratories
Fred Roozeboom, Universiteit Twente
Tadatomo Suga, Meisei University
Yu-Lin Wang, National Tsing Hua University
Energy Technology Division

Chair
Katherine E. Ayers, Nel Hydrogen
Vice Chair
Minhua Shao, Hong Kong University of Science and Technology
Secretary
Hui Xu, Envision Energy USA Treasurer
Gang Wu, University at Buffalo
Iryna Zenyuk, University of California, Irvine Member at Large
Christopher Arges, Pennsylvania State University
Plamen B. Atanassov, University of California, Irvine
Scott Calabrese Barton, Michigan State University
Rod Borup, Los Alamos National Laboratory
Nemanja Danilovic, Electric Hydrogen
Steven Decaluwe, Colorado School of Mines
Vito Di Noto, Università degli Studi di Padova
Huyen Dinh, National Renewable Energy Laboratory
James Fenton, University of Central Florida
Thomas Fuller, Georgia Institute of Technology
Andrew Herring, Colorado School of Mines
Ahmet Kusoglu, Lawrence Berkeley National Laboratory
Mani Manivannan, Global Pragmatic Materials
Sanjeev Mukerjee, Northeastern University
Sri Narayan, University of Southern California
Peter Pintauro, Vanderbilt University
Bryan Pivovar, National Renewable Energy Laboratory
Krishnan Rajeshwar, University of Texas at Arlington
Cynthia Rice, Plug Power, Inc.
Jacob Spendelow, Los Alamos National Laboratory
Jean St-Pierre, Cummins Technical Center
Vaidynathan Ravi Subramanian, University of Nevada, Reno
Adam Weber, Lawrence Berkeley National Laboratory
John Weidner, University of Cincinnati
Gang Wu, University at Buffalo
Nianqiang Nick Wu, University of Massachusetts Amherst
Thomas Zawodzinski, University of Tennessee, Knoxville
Iryna Zenyuk, University of California, Irvine
Organic & Biological Electrochemistry Division

Chair
Shelley Minteer, University of Utah
Vice Chair
Jeffrey Halpern, University of New Hampshire
2nd Vice Chair
Sabine Kuss, University of Manitoba
Secretary/Treasurer
Ariel Furst, Massachusetts Institute of Technology Member at Large
Mahito Atobe, Yokohama University
Mekki Bayachou, Cleveland State University
James Burgess, US Army Research Office
Graham Cheek, US Naval Academy
Dave Cliffel, Vanderbilt University
Robert Francke, Leibniz-Institut für Katalyse
Carlos Frontana-Vázquez, CIDETEQ
Ariel Furst, Massachusetts Institute of Technology
Matt Graaf, AbbVie, Inc.
Binbin Huang, Hunan University
Shinsuke Inagi, Tokyo Institute of Technology
Jiri Ludvik, J. Heyrovsky Institute of Physical Chemistry
Flavio Maran, Università degli Studi di Padova
Kevin Moeller, Washington University in St. Louis
Julie Renner, Case Western Reserve University
James Rusling, University of Connecticut
Lior Sepunaru, University of California, Santa Barbara
Charuksha Walgama, University of Houston-Clear Lake
Hai-Chao Xu, Xiamen University
24 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org
NEWS SOCIETY NEWS
SOCIETY
Physical & Analytical Electrochemistry Division
Chair
Stephen J. Paddison, University of Tennessee, Knoxville
Vice Chair
Anne Co, Ohio State University
Secretary
Svitlana Pylypenko, Colorado School of Mines
Treasurer
Iwona Rutkowska, Uniwersytet Warszawski
Member at Large
Robbyn Anand, Iowa State University
Plamen B. Atanassov, University of California, Irvine
D. Noel Buckley, University of Limerick
Abdoulaye Djire, Texas A&M University
Alanah Fitch, Loyola University
Burcu Gurkan, Case Western Reserve
David Hickey, Michigan State University
Yasushi Katayama, Keio University
Pawel J. Kulesza, Uniwersytet Warszawski
Johna Leddy, University of Iowa
Robert Mantz, US Army Research Office
Shelley Minteer, University of Utah
Hang Ren, The University of Texas at Austin
Joaquin Rodriguez López, University of Illinois at Urbana-Champaign
Alice Suroviec, Berry College
Greg Swain, Michigan State University
Paul Trulove, US Naval Academy
Petr Vanýsek, Northern Illinois University
Valentine Vullev, University of California, Riverside
Yingjie Zhang, University of Illinois at Urbana-Champaign
ECS Thanks Our 2022 Reviewers

The Electrochemical Society relies upon the technical expertise and judgment of the scientists who, by reviewing manuscripts, help to maintain the high standards characteristic of the Society’s peer-reviewed journals.


In 2022, more than 4,520 reviewers supported the Society’s long-standing commitment to ensuring both the technical quality of the work published and the integrity and validity of the peer-review process.

The Society would like to convey a sincere thank you to all of our reviewers for sharing their time and effort, and for their support of ECS and of the scientific process.
For a complete list of the reviewers of ECS journal articles in 2022, please visit the ECS News

Institutional Membership Program
Institutional membership provides organizations the opportunity to support and advance the dissemination of electrochemical and solid state science research. Member organizations save 15-20% in spending through discounts on ECS subscriptions, meeting registrations and marketing opportunities, and are able to provide ECS membership benefits to their employees.
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 25 SOCIETY NEWS SOCIETY NEWS
Read Online Now! ECS Sensors Plus is a one-stop shop journal for sensors. Gold Open Access. Read and publish for free in 2023 LEARN MORE
Contact Anna.Olsen@electrochem.org to learn more about institutional membership benefits.
Podcasts of Note
Suggested for you by Alice Suroviec.
Think: Sustainability Podcast Sustainability Defined


This weekly podcast, out of Sydney Australia, focuses on sustainability in a variety of ways. The impact of consumption in all aspects of life is examined. The host Marlene Even interviews different experts every week about current events such as carbon capture, resilient food systems, and sustainable fashion.
https://apple.co/2Bkn3Mv
How to Save a Planet
Alex Blumberg hosts this weekly podcast that focuses on climate change and the inspirational stories of people working to combat it. Each episode features a current topic with experts presenting their work in an accessible manner. Topics on this podcast range from deep sea mining to listener call-in shows.
https://gimletmedia.com/shows/howtosaveaplanet

This monthly podcast recognizes that sustainability as a topic is very broad and cannot be solved with a single approach. Each episode interviews an expert to understand the variety of ways sustainability affects our everyday life. These podcasts also come with notes for each episode with supplementary information. These podcasts have been used in a variety of classroom settings to help students engage with these important topics.
https://sustainabilitydefined.com
The Big Switch Podcast

Hosted by Dr. Melissa Lott, the research director at the Center on Global Energy Policy at Columbia University, this weekly podcast discusses how our current energy system needs to be rebuilt to address climate changes. These podcasts use historical and current events to put these large pressing questions into context.

https://www.energypolicy.columbia.edu/podcast/big-switch
© The Electrochemical Society. DOI: 10.1149/2.F03231IF
About the Author
Alice Suroviec is Professor of Bioanalytical Chemistry and Dean of the College of Mathematical and Natural Sciences at Berry College. She earned a BS in Chemistry from Allegheny College in 2000. She received her PhD from Virginia Tech in 2005 under the direction of Dr. Mark R. Anderson. Her research focuses on enzymatically modified electrodes for use as biosensors. She is currently Associate Editor of the PAE Technical Division for the Journal of the Electrochemical Society. She is always looking for new app/podcast/website suggestions, so feel free to email her.
https://orcid.org/0000-0002-9252-2468
26 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org SOCIETY NEWS SOCIETY NEWS
PARTNERSHIPS ACCELERATE WITH

ECS. Connect with us in 2023!
International Battery Seminar & Exhibit (IBSE)
Booth #317
March 20-23 l ORLANDO, FL, USA
American Chemical Society (ACS)
August 13-17 l SAN FRANCISCO, CA, USA
International Society of Electrochemistry (ISE)
September 3-8 l LYON , FRANCE
American Institute of Chemical Engineers (AIChE)
November 5-10 l ORLANDO, FL, USA
Materials Research Society (MRS)
November 26-December 1 l BOSTON , MA, USA
Advanced Automotive Battery Conference US (AABC)
December 5-8 l SAN DIEGO, CA, USA
UPCOMING ECS SPONSORED MEETINGS
In addition to ECS biannual meetings and satellite conferences, the Society, ECS divisions, and ECS sections sponsor meetings and symposia of interest to the technical audience ECS serves. Here is a partial list of upcoming sponsored meetings. Visit the ECS website for a list of all sponsored meetings
2023
StorageX International Symposium Series
Ongoing Fridays – Virtual lectures
Stanford University
Dennis G. Peters Memorial Symposium
April 15, 2023 – In-person and virtual

Indiana University Bloomington
18th International Symposium on Solid Oxide Fuel Cells (SOFC-XVIII) with the 243rd ECS Meeting
May 28–June 2, 2023 – Boston, MA
Hynes Convention Center and Sheraton Boston
1st International Workshop of the Bioelectrochemical Society
June 14–16, 2023
University of Utah
2025
19th International Symposium on Solid Oxide Fuel Cells (SOFC-XIX)
July 13–18, 2025 – Stockholm, Sweden
The Brewery Conference Center
For information on the benefits of ECS meeting sponsorship (including publishing sponsored meetings’ proceedings volumes), or to request ECS sponsorship for your technical event, contact ecs@electrochem.org.
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 27 SOCIETY NEWS SOCIETY NEWS
LEARN MORE: Contact sponsorship@electrochem.org!
JOB
POSTING PACKAGES JOB FLASH™
ECS Career Center
Is the premier electronic provider of career and talent management resources for electrochemistry and solid state science professionals. Employers can connect to both active and passive job seekers who seek new career opportunities.

USER-FRIENDLY INTERFACE
Quick and easy account registration and management with logo upload capabilities and detailed company profile.
EXCLUSIVE MEMBER BENEFIT
ECS members save up to $100 on certain job posting packages.
JOB POSTING OPTIONS
The ECS Career Center offers a variety of job posting options, including basic, enhanced, and passive job seeker postings.
JOB FLASH™ EMAIL
Email your job to thousands of electrochemical and solid state science professionals with this special feature.
RESUME BANK
28 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org Contact ECS Career Center ’s customer service department at clientserv@communitybrands.com or Call (727) 497-6565 for more information. jobs.electrochem.org
EMAIL BANNER
The Resume Bank allows the ability to contact potential candidates, only paying for those who are interested. RESUME
ADVERTISING
BANK ACCESS
EMPLOYER BENEFITS:
In Memoriam ... Diane

K. Smith
1960–2022
Diane K. Smith passed away on October 24, 2022, in her San Diego home, from complications of scleroderma. She was a distinguished scientist, gifted teacher, and active member of ECS in the Organic and Biological Electrochemistry Division for more than 30 years.
Diane was born to Janet Kimball Smith and J. Leonard Smith and raised in Newport Beach, California. She was studious, inquisitive, active in Girl Scouts, played flute, and developed an interest in painting and the natural environment. She graduated from Newport Harbor High School in 1978 and Lewis and Clark College in 1982.
She received a scholarship to Massachusetts Institute of Technology. There she developed her knowledge, experience, and many friendships in Boston, graduating with her PhD in Chemistry in 1988. She joined the Postdoctoral program at University of Delaware and in 1990 was the first woman hired into a tenure track position in the Chemistry Department at San Diego State University.
Diane was an inspiration to women pursuing careers in science throughout her 30 years at SDSU. She was a dedicated teacher who taught many beginning and advanced chemistry courses. Her enthusiastic instruction and caring guidance touched the lives of numerous students, influencing several to choose to major in chemistry and preparing undergraduate and graduate students alike
to pursue careers in academia and industry. She served as the Chair of the Department’s Curriculum Committee for decades, advocating for the university’s educational mission overall, and the master’s in chemistry program in particular.
In 2022, Diane was awarded the Jaroslav Heyrovsky Prize for Molecular Electrochemistry by the International Society of Electrochemistry. She was nominated and selected because of her illustrious career investigating methods to couple electron and proton transfer in reversible organic redox reactions. Her work informed the development of drugs to fight anaerobic microbial infections, among other advancements in the field.
Diane was a dedicated volunteer to ECS and the science, serving in many roles:
• Member-At-Large, Sensor Division, 2001–2004
• Secretary/Treasurer, Organic and Biological Electrochemistry Division, 2015–2017
• Vice Chair, Organic and Biological Electrochemistry Division, 2017–2019
• Chair, Organic and Biological Electrochemistry Division, 2019–2021
• Member, ECS Board of Directors, 2019–2021

• Member, Interdisciplinary Science and Technology Subcommittee, 2018–2022
• Member, Honors and Awards Subcommittee, 2020–2022
• Member, Acheson Award Selection Subcommittee, 2021–2022
• Member, John B. Goodenough Award Subcommittee, 2022.
This notice is based on remembrances published by SDSU and by Diane’s family.
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 29 SOCIETY NEWS SOCIETY NEWS PEOPLE NEWS PEOPLE NEWS ECS WEBINAR SERIES Upcoming and past webinars are at ELECTROCHEM.ORG/WEBINARS Showcasing distinguished speakers and ECS community members Co-sponsored by ECS and our PhysicsWorld.com partners
ACCELERATE
243rd ECS Meeting
Short Courses
May 28, 2023:
Boston, MA
Basic Impedance Spectroscopy
Mark Orazem, Instructor
Fundamentals of Electrochemistry: Basic Theory and Thermodynamic Methods
James Noël, Instructor
Lithium-Ion Battery Safety and Failure Modes
Thomas Barrera, Instructor
Electrochemical Capacitor Technology
John Miller, Instructor
Courses open to everyone— students, mid-career and seasoned professionals, industry!

244th ECS Meeting
Short Courses
October 8, 2023:
Gothenburg, Sweden
Advanced Impedance Spectroscopy
Mark Orazem, Instructor
Fundamentals of Electrochemistry: Basic Theory and Kinetic Methods
James Noël, Instructor
Lithium-Ion Battery Safety and Failure Modes
Thomas Barrera, Instructor
Electrodeposition for Energy Applications
Stanko Brankovic and Sudipta Roy, Instructors
Intensive in-person instruction by topic experts. Register for as little as $375! Learn
more
electrochem.org/short-courses!
POTENTIAL with
at
YOUR
ECS Short Courses!
Looking at Patent Law: Patenting an Iron Slurry Electrode Redox Flow Battery – A Case Study
by E. Jennings Taylor and Maria Inman
In this installment of the ‟Looking at Patent Lawˮ articles, we present a case study of a patented invention of an iron slurry electrode redox flow battery. This invention aligns with several divisions of the Electrochemical Society (ECS), including Industrial Electrochemistry and Electrochemical Engineering (IE&EE), Battery (BATT), Energy Technology (ETD), and Electrodeposition (ELDP).
Recall from our previous article,1 the prosecution history (i.e., examination record) of a patent application is publicly available in the file wrapper of the United States Patent and Trademark Office (USPTO) Patent Center.2 With the USPTO system as the primary source of information for this case study, we illustrate the prosecution “events” encountered during the examination of US Patent No. 9,559,375: “Iron Flow Batteries.”3 The ‘375 patent issued on January 31, 2017 with inventors Robert F. Savinell and Jesse S. Wainright. Dr. Savinell is a Distinguished University Professor and Professor of Chemical Engineering at Case Western Reserve University (CWRU). Dr. Wainright is Research Professor of Chemical Engineering at CWRU. The assignee of the patent at the time of issue was CWRU.
Dr. Savinell has been a member of the ECS since 1978 and is a frequent presenter at ECS biannual meetings. Dr. Savinell is Past Chair of the IEEE Division. He was elected ECS Fellow in 2000 and was the recipient of the ECS Vittorio de Nora Award in 2022. Dr. Savinell currently serves as Editor-in-Chief of the Journal of The Electrochemical Society. Dr. Wainright is a long-time member of the ECS and a frequent presenter at ECS biannual meetings.
A prototype ten-cell stack iron slurry electrode redox flow battery is pictured in Fig. 1.4 Inventors Savinell and Wainright are pictured with the prototype flow battery along with PhD student Nick Sinclair. The iron slurry electrode redox flow battery was described in a journal publication after the priority date of the subject patent application.5 The issues associated with scale-up of the novel redox flow battery have been recently described6 and the technical and non-technical issues culminating with the licensing of the subject technology is the subject of a presentation at the 243rd ECS Meeting in Boston in 2023 (R. Savinell, J. Wainright, N. Sinclair, “Iron Flow Battery with Slurry Electrode for Large Scale Energy Storage: Scale-up, Commercialization, and IP Challenges in an Academic Environment”). A key innovation of the invention is the use of a slurry consisting of conducting particles for iron plating and deplating.

The ‘375 patent abstract generally describes the invention as follows:
“An iron based redox flow cell. The redox flow cell comprises a first half-cell comprising a first electrolyte providing a source of Fe2+ ions and an electrode disposed within the first half-cell; a second half-cell comprising a second electrolyte providing a source of Fe2+ and Fe3+ ions and an electrode disposed within the second half-cell; and a separator between the first and second half-cells, where (a) the second electrolyte comprises a Fe3+ stabilizing agent; (b) the first electrolyte comprises a hydrogen evolution suppressing agent; or (c) the first electrolyte comprises a hydrogen evolution suppressing agent, and the second electrolyte comprises a Fe3+ stabilizing agent.”
(continued on next page)
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 31
+ -
Fig. 1. Picture of the Iron Slurry Electrode Redox Battery with Prof. Robert Savinell (left), PhD Student Nick Sinclair (center), and Research Prof. Jesse Wainright (right).
Fig 1. Picture of Iron Slurry Electrode Redox Battery with Prof. Robert Savinell (left), PhD Student Nick Sinclair (center) and Research Prof. Jesse Wainright (right).
Patent Applications
This article summarizes the prosecution history of Patent Application No. 14/122,885 (filed on June 1, 2012), leading to US Patent No. 9,559,375. In Table I, we list the patent applications related to Patent Application No. 14/122,885. US Provisional Patent Application No. 61/491,973 and Patent Cooperation Treaty (PCT) Application No. PCT/US2012/040429 were filed on June 1, 2011. A claim is not required in a provisional patent application7 and a provisional patent application expires one year from its filing date.8 Consequently the US utility and PCT patent applications were filed within one year of the filing date of the provisional patent application. The priority date of the US utility and PCT patent applications is the filing date of the US provisional patent application: June 1, 2011.9 The iron redox battery development team used the provisional patent application as a placeholder while additional experimental activities progressed.
Description of the Invention
The “Background” section of the patent application describes a reduction-oxidation flow battery as

“…an electrochemical storage device in which an electrolyte containing one or more dissolved electro-active species flows through a reactor cell where chemical energy is converted to electrical energy… The electrolytes used in flow batteries are generally composed of ionized metal salts that are stored in large external tanks and are pumped through each side of the cell according to the charge/discharge current applied. Externally stored electrolytes can be flowed through the battery system by pumping, gravity feed, or by any other method of moving fluid through the system. The reaction in a flow battery is reversible, and the electrolyte can be recharged without replacing the electroactive material. The energy capacity of a redox flow battery, therefore, is related to the total electrolyte volume, e.g., the size of the storage tank. The discharge time of a redox flow battery at full power also depends on electrolyte volume and often varies from several minutes to many days.”
FIG. 1 from the “Detailed Description” section of the patent application is reproduced in Fig. 2. Flow cell 100 includes two halfcells 102 and 104 separated by a separator 106. Half cells 102 and 104 include electrodes 108 and 110, respectively, in contact with an electrolyte such that an anodic reaction occurs at the surface of one of the electrodes and a cathodic reaction occurs at the other electrode. Electrolyte flows through each of the half-cells 102 and 104 as the oxidation and reduction reactions take place. The cathodic reaction takes place in half-cell 102 at electrode 108 (which is referred to herein as the positive electrode or the cathode), and the anodic reaction takes place in half-cell 104 at electrode 110 (which is referred to herein as the negative electrode or the anode).
The electrolyte in half-cells 102 and 104 flows through the system to storage tanks 112 and 114, respectively, and fresh/regenerated electrolyte flows from the tanks back into the half-cells. The electrolyte
in half-cell 102 flows through pipe 116 to holding tank 112, and the electrolyte in tank 112 flows to the half-cell 102 through pipe 118 Similarly, the electrolyte in half-cell 104 can flow through pipe 120 to holding tank 114, and electrolyte from tank 114 flows through pipe 122 to half-cell 104. The systems can be configured as desired to aid or control the flow of electrolyte through the system and may include, for example, any suitable pumps or valve systems. In the embodiment depicted in FIG. 1, the system includes pumps 124 and 126 to pump the electrolyte from tanks 112 and 114, respectively, to the half-cells. In some embodiments, the holding tank can segregate electrolyte that has flowed through the respective cells from electrolyte that has not. However, mixing of discharged or partially discharged electrolyte can also be performed.
Electrodes 108 and 110 can be coupled to either supply electrical energy or receive electrical energy from a load or source. Other monitoring and control electronics included in the load can control the flow of electrolyte through half-cells 102 and 104. A plurality of cells 100 can be electrically coupled (“stacked”) in series to achieve higher voltage or in parallel to achieve higher current.
In the iron flow battery, the half-cell reactions are as follows:
Charge:
Fe2++2e →Fe0 Negative Electrode
2Fe2+→2Fe3++2e Positive Electrode
Discharge:
Fe0→Fe2++2e Negative Electrode
2Fe3++2e →2Fe2+ Positive Electrode
Iron plates out (iron plating 128) onto the negative electrode 110 in half-cell 104 during charging and Fe2+ is released upon discharge.
The electrolytes for the half-cells 102 and 104 are chosen to provide a suitable source of the ions required to carry out the reactions in each half-cell. The electrolyte used for the redox reactions at the positive electrode is a suitable salt solution comprising a source of ferrous (Fe2+) and ferric (Fe3+) ions. This electrolyte is also referred to herein as the catholyte. The electrolyte used for the reactions at the negative electrode comprises a source of Fe2+ ions. This electrolyte is also
32 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org
Fig. 2. Battery.
Fig 2. FIG. 1 from the ‘375 Patent Illustrating the Iron Redox Flow Battery. Taylor and Inman (continued from previous page)
APPL. TYPE APPL. No. PAT. No. TITLE FILING DATE Status U.S. Provisional 61/491,973 N/A Iron Based Flow Batteries Jun. 1, 2011 Expired U.S Utility 14/122,885 9,559,375 Iron Based Flow Batteries Jun. 1, 2012 Issued Jan. 31, 2017 PCT US2012/ 040429 Various Iron Based Flow Batteries Jun. 1, 2012 Issued Issued Various Foreign Countries
Table I. Patent applications associated with the iron slurry electrode redox battery.
referred to herein as the anolyte. The electrolyte may be any suitable salt, including, but not limited to, the chloride, sulfate, or nitrate salts or a combination of two or more thereof. In one embodiment, the catholyte comprises a solution of FeCl2 and FeCl3, which provides a relatively large charge carrying capacity compared to other iron salts. The concentration of the salt providing the Fe2+ ions (e.g., FeCl2) may be from about 0.01 M to about 5 M, about 0.05 M to about 2.5 M, and even about 0.1 M to about 1 M, and the concentration of the salt providing the Fe3+ ions (e.g., FeCl3) may be from about 0.01 to about 5 M, about 0.05 M to about 2.5 M, and even about 0.1 M to about 1 M. In one embodiment, the concentration of the FeCl2 is about 1.0 M, and the concentration of the FeCl3 is about 1.0 M.
The “Detailed Description” continues
“In one embodiment a system for decoupling the power/ energy at the anode comprises employing a slurry electrode or fluidized bed electrode as the negative electrode. The slurry comprises particles sufficient to impart electrode conductivity to the electrolyte. Suitable particles include carbon-based, e.g., graphitic particles, iron particles, iron coated particles, or a combination of two or more thereof. The iron coated particles can include an electrically conductive particle as the core. In one embodiment, the iron coated particles comprise carbon-based particles, copper particles, or titanium particles coated with iron. The iron coated particles can be particles comprising iron plating. Over time, the iron particles and iron coating can be depleted, and the use of iron coated particles provides a slurry that still exhibits electrical conductivity via the electrically conductive particles. In one embodiment, a slurry electrode comprises iron particles suspended in a sufficient volume of electrolyte to enable the slurry to be pumped through the battery, while still maintaining particle to particle contact for electrical conductivity. The particle size can be chosen as desired. In one embodiment, the particles can have a particle size of from about 1 micron to about 1500 microns; from about 5 microns to about 1000 microns; from about 10 microns to about 500 microns; from about 20 microns to about 250 microns; even from about 50 microns to about 100 microns. In one embodiment, the particles have an average particle size of about 100 microns. Here as elsewhere in the specification and claims numerical values can be combined to form new or non-disclosed ranges. Without being bound to any particular theory, using larger particles may reduce particle to particle contacts and increase the conductivity of the slurry. Using a slurry electrode provides a high surface area to minimize the over potential for iron plating/dissolution and a higher cycle life (compared to plating on a flat electrode).”
Establishing and Maintaining a Filing Date
In order to establish a filing date, a utility patent application must include
1) Specification10
“…a written description of the invention, and the manner and process for making it…to enable any person skilled in the art…to make and use [the invention] …”
2) Minimum of one claim11
“…particularly pointing out…the subject matter…as the invention…”
3) Drawings12
“…where necessary for understanding the subject matter…to be patented…”
In order to maintain the filing date, the following additional criteria are required
1) Filing fee in accordance with the current USPTO fee schedule13
2) Inventor oath or declaration asserting14
a) The patent application was authorized by the inventor(s),
b) The inventor(s) believe he/she is the original inventor or they are the original joint inventors.
The PCT patent application was filed on June 1, 2012 electing the US and other foreign jurisdictions. The patent application included
1) specification,
2) claims,
3) drawings, and
4) filing fee.
As summarized above, the specification included a background of the invention and a summary of the invention describing various embodiments of the invention. Additionally, the patent application also included drawings illustrating the “elements” and various embodiments of the subject invention. The utility patent application contained claims directed toward the iron redox flow battery.
The US Patent & Trademark Office (USPTO) issued a “Notification of Missing Requirements” for the applicants to file an Oath or Declaration. In response to the USPTO “Notice,” the applicants submitted a declaration signed by each of the inventors.15 The declaration included an assertion by the inventors stating,
“I hereby state that I have reviewed and understand the contents of the above-identified specification, including the claims,…I acknowledge the duty to disclose to the United States Patent and Trademark Office all information known to me to be material,…I hereby claim the benefit…of any PCT International application designating the United States…”
The declaration also included an acknowledgment that each inventor was aware of the penalties for a false statement,16
“I hereby declare that all statements made herein of my own knowledge are true and that all statements are made on information and belief are believed to be true; and further that these statements were made with the knowledge that willful false statements and the like so made are punishable by fine or imprisonment, or both, under Section 1001 of Title 18 of the United States Code and that such willful false statements may jeopardize the validity of the application or any patent issued thereon”
Importantly, the “named inventors” must be correctly represented on a US patent application.17 Specifically, inclusion of a colleague as a co-inventor who did not participate in the conception of the invention is known as a misjoinder and may invalidate an otherwise valid patent. Similarly, exclusion of a co-inventor who participated in the conception is known as a nonjoinder and may invalidate an otherwise valid patent. If an inventor is erroneously omitted or erroneously included as an inventor, the misjoinder/nonjoinder may be corrected and the patent remains valid.18
The filing of the patent application and the subsequently submitted declaration met the requirements to both establish and maintain a filing date and thereby avoided being abandoned.
The USPTO began publishing patent applications eighteen months after their priority date for patent applications filed on or after November 29, 2000. Since the subject patent application was filed after this date it was published.
(continued on next page)
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 33
Inventor Assignment, Small Entity Status, and Power of Attorney
The inventors were employed by Case Western Reserve University (CWRU) and the patent application was assigned to CWRU.19 The patent application included a statement asserting “small entity” status as the assignee was a nonprofit university.20 The small entity status entitled the applicants to reduced filing, issue, and maintenance fees. In addition, the applicants appointed patent attorneys/agents from the law firm McDonald Hopkins LLC to prosecute the patent application at the USPTO.
March-In Rights
The work leading to the subject invention was supported with funding from the DOE Advanced Research Projects Agency (ARPA-E). The Bayh-Dole Act stipulates that an invention made with government funding include a government rights statement.21 Although the patent application did not include a government rights statement, a “Certificate of Correction” was submitted by the applicants after the patent issued correcting the omission
“This invention was made with government support under government Contract No. DE-AR0000352 awarded by The United States Department of Energy. The government has certain rights in the invention.”
Regarding march-in rights, a key policy objective of the Bayh-Dole Act is22
“…to ensure that the Government obtains sufficient rights in federally supported inventions to meet the needs of the Government and protect the public against nonuse or unreasonable use of inventions…”
To our knowledge, the government has never exercised Bayh-Dole march-in rights in any invention.
Information Disclosure Statement
The applicants submitted an “Information Disclosure Statement” (IDS) to the USPTO with the patent application. Updated IDSs were submitted during the prosecution of the patent application. The IDS included prior art references, including those of the inventors, as required by the “Duty of Candor.” The “Duty of Candor” requires that the inventor(s) submit an IDS within a reasonable time after the submission of the patent application, disclosing23
the Office [USPTO] all information known to that individual to be material to patentability…”
The “Duty of Candor” is specific to any existing claim and requires that the IDS be continually updated while the claim is pending. The “Duty of Candor” ceases only when the claim is allowed and the patent issue fee is paid.
The “Duty of Candor” extends to any individual associated with the filing of the patent application, including
1) Inventor(s),
2) Patent Counsel, or
3) Persons who are substantially involved in the preparation or prosecution of the patent application.
Substantial involvement in the preparation of the patent application could include technical assistants, collaborators, or colleagues. Substantial involvement would generally not extend to clerical workers. Furthermore, the inclusion of a reference in an IDS24
“…is not taken as an admission that the reference is prior art against the claims.”
If a finding of a violation of the “Duty of Candor” resulting in “inequitable conduct” regarding any claim in a patent is determined, then all the claims of the subject patent are rendered invalid.25 Finally, in spite of the requirement of the “Duty of Candor,” the applicant is cautioned not to “bury” the examiner with a long list of non-material references in hopes that the examiner will not notice the relevant material references.26 The specific guidance from the USPTO is to27
“…avoid the submission of long lists of documents if it can be avoided…If a long list is submitted, highlight those documents which have been specifically brought to the applicant’s attention and/or are known to be of most significance.”
Claims
Independent Claim 1 from the patent application is reproduced herein.
Claim 1. An iron flow redox cell comprising:
a first half-cell comprising a first electrolyte providing a source of Fe2+ ions and an electrode disposed within the first halfcell;
a second half-cell comprising a second electrolyte providing a source of Fe2+ and Fe3+ ions and an electrode disposed within the second half-cell;
a separator between the first and second half-cells;
a first storage tank external to the first half-cell for circulating the first electrolyte to and from the first half-cell; and
a second storage tank external to the second half-cell for circulating the second electrolyte to and from the second half-cell;
the half-cells conducting an oxidation reduction reaction to charge and discharge the battery, wherein
(a) the second electrolyte comprises a Fe3+ stabilizing agent;
(b) the first electrolyte comprises a hydrogen evolution suppressing agent; or
(c) the first electrolyte comprises a hydrogen evolution suppressing agent, and the second electrolyte comprises a Fe3+ stabilizing agent.
Additionally, Dependent Claim 14 is reproduced herein.
Claim 14. The iron flow redox cell of any of claims 1 – 13, wherein the electrode in the first half-cell comprises a slurry comprising electrically conductive particles, iron particles, iron coated particles, or a combination thereof.
Non-Final Office Action
On March 10, 2016, the USPTO issued a non-final office action (NF-OA) rejecting all the claims in the patent application based on “indefiniteness”28
“…as being indefinite for failing to specifically point out and distinctly claim the subject matter which applicant regards as the invention.”
or lack of “novelty” in view of the prior art29
“…the invention was patented or described in a printed publication in this or a foreign country or in public use or on sale in this country, more than one year prior to the date of application for patent in the United States.”
34 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org
“…to
Taylor and Inman (continued from previous page)
or as being “obvious” in view of the prior art30
“A patent may not be obtained…if the differences between the subject matter sought to be patented and the prior art are such that the subject matter as a whole would have been obvious at the time the invention was made to a person having ordinary skill in the art...”
Both the lack of “novelty” and “obviousness” rejections cited a prior publication in JES by one of the inventors in 1981.31 This prior art publication was included in the IDS. While inventors may consider not disclosing their own prior publications to the USPTO in the hope that the examiner does not discover them, this would not be good practice. Specifically, not disclosing prior publications by the inventor would likely be a breach of the “Duty of Candor” and when discovered could render the patent application invalid.
The applicants were given a three-month period to respond without paying additional late response fees.
Applicant Response
On June 10, 2016, the applicants responded within the threemonth period set by the USPTO to avoid late fees. The inventors addressed the “indefiniteness” rejection by editing the claims. The inventors addressed the lack of “novelty” and “obviousness” rejections by amending Independent Claim 1 to include the limitation from Dependent Claim 14. The amended Claim 1 is reproduced herein with the inserted text depicted in [brackets].
Claim 1. An iron flow redox cell comprising:
a first half-cell comprising a first electrolyte providing a source of Fe2+ ions and an electrode disposed within the first half-cell;
a second half-cell comprising a second electrolyte providing a source of Fe2+ and Fe3+ ions and an electrode disposed within the second half-cell;
a separator between the first and second half-cells; a first storage tank external to the first half-cell for circulating the first electrolyte to and from the first half-cell; and a second storage tank external to the second half-cell for circulating the second electrolyte to and from the second half-cell, the half-cells conducting[;]
[wherein the first and second half-cells conduct] an oxidation reduction reaction to charge and discharge the battery[iron flow redox cell], wherein
(a) the second electrolyte comprises a Fe3+ stabilizing agent;
(b) the first electrolyte comprises a hydrogen evolution suppressing agent; or
(c) the first electrolyte comprises a hydrogen evolution suppressing agent, and the second electrolyte comprises a Fe3+ stabilizing agent; [and]
[wherein the electrode in the first half-cell comprises an aqueous slurry comprising electrically conductive particles, iron particles, iron coated particles, or a combination thereof].
Allowance of Patent Application
On September 28, 2016, the USPTO issued a notice of allowance. The amended Independent Claim 1 allowed the applicants to overcome the “lack of novelty” and “obviousness” rejections in the NF-OA. Additionally, all the dependent claims were allowed. After payment of the issue fees, the patent application was issued as US Patent No. 9,559,375 on January 31, 2017.
Summary
In this installment of our “Looking at Patent Law” series, we present a case study of the prosecution of US Patent No. 9,559,375: “Iron Flow Battery.” This invention aligns with several ECS divisions, including Industrial Electrochemistry and Electrochemical Engineering (IE&EE), Battery (BATT), Energy Technology (ETD), and Electrodeposition (ELDP). The ‘375 patent issued on January 31, 2017 with inventors Robert F. Savinell and Jesse S. Wainright. The assignee of the patent was Case Western Reserve University (CWRU). The case study begins with a brief synopsis of the background of the invention followed by 1) summary of key drawings and the specification of the invention, 2) inventor assignment and power of attorney designations, 3) submission of the Invention Disclosure Statement (IDS) and associated Duty of Candor, 4) summary of the non-final office action (NF-OA) and rejection, and 5) applicant response and allowance of the patent application. The case study illustrates overcoming “lack of novelty” and “obviousness” rejections by combining the limitation of dependent claims with the independent claim. With this case study, we hope to de-mystify the patent prosecution process and better prepare electrochemical and solid-state scientists, engineers, and technologists to interact with their patent counsel regarding their inventions.
© The Electrochemical Society. DOI: 10.1149/2.F04231IF
About the Authors
E. Jennings Taylor, Founder of Faraday Technology, Inc.
Research Interest: Faraday Technology, Inc. is a small business focused on developing innovative electrochemical processes and technologies based on pulse and pulse reverse electrolytic principles.
Patent Background: Taylor leads Faraday’s patent and commercialization strategy and has negotiated numerous patents via field of use licenses as well as patent sales. He is admitted to practice before the United States Patent & Trademark Office (USPTO) in patents cases as a patent agent (Registration No. 53,676). Member of the American Intellectual Property Law Association (AIPLA).
Pubs & Patents: Numerous technical pubs and presentations, inventor on 40 patents.
Work with ECS: Member for 42 years, ECS Fellow.
Website: http://www.faradaytechnology.com/ https://orcid.org/0000-0002-3410-0267
Maria Inman, Research Director, Faraday Technology, Inc.
Patent Background: Inman serves as principal investigator on project development activities and manages the company’s pulse and pulse reverse research project portfolio.
Pubs & Patents: In addition to technical pubs and presentations, she is competent in patent drafting and patent drawing preparation. She is an inventor on seven patents.
Work with ECS: Member for 25 years. Chair, Industrial Electrochemistry and Electrochemical Engineering Division. Serves ECS as a member of many committees.


Awards: ASTM member
Website: http://www.faradaytechnology.com/ https://orcid.org/0000-0003-2560-8410
(continued on next page)
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 35
Taylor and Inman
(continued from previous page)
References
1. E. J. Taylor and M. Inman, Electrochem Soc Interface, 26 (4), 57 (2017).
2. Patent Center https://patentcenter.uspto.gov
3. R. F. Savinell and J. S. Wainright,” Iron Flow Batteries” U.S. Patent No. 9,559,375 issued January 31, 2017.
4. https://thedaily.case.edu/researchers-building-flow-batteryprototype-augment-grid/ (accessed 12/6/2022)

5. T. J. Petek, N. C. Hoyt, R. F. Savinell, and J. S. Wainright, J. Power Sources, 294, 620 (2015)
6. N. S. Sinclair, R. F. Savinell, and J. S. Wainright, MRS Energy Sustain, 20, (2022)
7. 35 U.S.C. §111(b)(2) Provisional Application/Claim.
8. 35 U.S.C. §111(b)(5) Provisional Application/Abandonment.
9. 35 U.S.C. §119(a) Benefit of Earlier Filing Date; Right of Priority.
10. 35 U.S.C. §112(a) Specification/In General.
11. 35 U.S.C. §112(b) Specification/Conclusion.
12. 35 U.S.C. §113 Drawings.
13. https://www.uspto.gov/learning-and-resources/fees-andpayment/uspto-fee-schedule#Patent%20Fees
14. 35 U.S.C. §115(b)(1)(2) Inventor’s Oath or Declaration/ Required Statements.
15. 37 CFR 1.63 Inventor’s Oath or Declaration.
16. 18 U.S.C. §1001Statements or Entries Generally.
17. E. J. Taylor and M. Inman, Electrochem Soc Interface, 26 (2), 45 (2017).
18. Manual of Patent Examination Procedure (MPEP) §1481.02 Correction of Named Inventor.
19. 35 U.S.C. §261 Ownership; Assignment.
20. 37 CFR §1.27(a)(3)(ii)(A) Definition of small entities and establishing status as a small entity to permit payment of small entity fees; when a determination of entitlement to small entity status and notification of loss of entitlement to small entity status are required; fraud on the Office.
21. 35 U.S.C. §203 March-in Rights.
22. 35 U.S.C. §200 Policy and Objective.
23. 37 CFR §1.56(a) Duty to Disclose Information Material to Patentability.
24. Riverwood Int’l Corp. v. R.A. Jones & Co., 324 F.3d 1346, 135455, 66 USPQ2d 1331, 1337-38 (Fed Cir. 2003).
25. Manual of Patent Examination Procedure (MPEP) §2016 Fraud, Inequitable Conduct, or Violation of Duty of Disclosure Affects All Claims
26. R. B. Taylor, Mich. Telecomm. & Tech. Law Rev., 99, 19 (2012).
27. Manual of Patent Examination Procedure (MPEP) §2004.13 Aids to Comply with Duty of Disclosure.
28. 35 U.S.C. §112(2) (pre-AIA) Specification.
29. 35 U.S.C. §102(b) (pre-AIA) Conditions for Patentability; Novelty and Loss of Right to Patent.
30. 35 U.S.C. §103(a) (pre-AIA) Conditions for Patentability; Nonobvious Subject Matter.
31. L. W. Hruska and R. F. Savinell, J Electrochem Soc, 128, 18 (1981).
36 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org
with the 18th International Symposium on Solid Oxide Fuel Cells (SOFC-XVIII) Registration is currently Open Early registration deadline is April 24, 2023 REGISTER NOW BOSTON, MA May 28-June 2, 2023
243rd ECS MEETING
Reports from the Frontier
edited by Scott Cushing, Interface Contributing Editor


This feature is intended to let ECS award-winning students and post-docs write a primaryauthor perspective on their field, their work, and where they believe things are going. This month we highlight the work of Zachary J. Schiffer, a 2022 winner of the ECS Energy Technology Division Graduate Student Award Sponsored by BioLogic.
Heterogeneous Electrocatalysts for Sustainable Electrochemical Synthesis
by Zachary J. Schiffer
Electrification and Decarbonization of Chemical Industry
Chemical synthesis impacts a range of industries, including energy, transportation, construction, and medicine, and it plays a role in the production of more than 95% of manufactured goods.1 In total, chemical manufacturing uses around 10% of worldwide energy demand and generates 7% of global greenhouse gas emissions.1 The chemical industry is therefore a prime target for global decarbonization and the reduction of greenhouse gas emissions.2,3 While many largevolume processes such as the production of ethanol, ammonia, and chlorine individually consume significant global energy demand and are attractive targets for decarbonization, the subsector representing basic organic chemicals has the largest onsite energy consumption within the chemical manufacturing sector in the United States (20% of the sector, Fig. 1a), more than the petrochemical subsector (18%), the plastics materials subsector (14%), and the nitrogenous fertilizer subsector (5%). Accordingly, decarbonizing the manufacturing of basic organic chemicals is an important and necessary step for reducing emissions from the chemical industry.4
One key strategy toward decarbonizing chemical synthesis is to reduce reliance on fossil fuels as an energy source. The increased availability of renewable electricity from sources such as solar and wind offers opportunities to both reduce reliance on fossil fuels and electrify chemical manufacturing.2,5 While there are many possible uses for renewable electricity, such as joule heating of reactors, one approach is to use these electrons to make and break chemical bonds directly via electrochemistry. Electrochemical manufacturing has historically been limited to a few processes, such as the chlor-alkali and aluminum industries, but with access to cheap, renewable electricity and improved tools for synthesizing catalysts, electrochemistry offers a powerful driving force for a wide range of chemical transformations.3,5,6 Specifically, electrochemical devices can avoid energy-intensive reactors that operate at high
temperatures and pressures while also potentially reducing expensive separation processes. Additionally, electrochemical devices do not rely on stoichiometric redox reagents, improving reactor safety and minimizing waste. For electrochemical processes to become practical, however, electrocatalysts are necessary to reduce energy requirements and tune reaction selectivity.
Heterogeneous Catalysts in Traditional Industry
A consistent goal of the chemical industry is energy efficiency, both because reduced energy consumption leads to cheaper processes and because more efficient processes results in fewer emissions. One major route toward improved energy efficiency and decarbonization is the development of new catalytic processes.1 Catalysts directly lower energy barriers in chemical transformations and bring energy requirements closer to their theoretical minima. Unsurprisingly, 85 to 90% of chemical processes currently rely on catalysts.7 In brief, there are three major types of catalysts widely employed: enzymatic, homogeneous, and heterogeneous catalysts. Enzymatic catalysts are generally favored when high chemo-, regio-, and enantioselectivity are essential (e.g., in the production of certain biologically relevant molecules). Homogeneous catalysts, such as organometallic complexes, are used in reactions such as carbonylations, hydroformylations, and cross-coupling reactions but can be problematic in continuous processes due to the necessary separation from the product for reuse. Heterogeneous catalysts are widely used in the chemical industry due to their ease of recovery and recycling, their utility in scalable continuous systems, and the availability of extensive advanced tools for their synthesis and characterization.8 While there are many arguments for each type of catalyst, heterogeneous catalysts match well with the requirements for industrial electrochemical manufacturing because of the ease of recycling and recovery as well as the scalability of high surface area heterogeneous catalysts on electrode surfaces.
(continued on next page)
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 37
(D) Depiction of some recent work on inner-sphere heterogeneous electrochemical syntheses with catalysts indicated.17,18,20
Current Electrocatalytic Systems
Recent advances in organic electrochemistry have generally relied on either outer-sphere electron transfers, such as on glassy carbon electrodes, or homogeneous redox mediators, such as organometallic homogeneous catalysts.9–12 In outer-sphere systems, optimizing selectivity and reducing energy efficiency is difficult without a catalyst; the choice of solvent and additives is the main method for controlling reactivity. Homogeneous redox mediators such as organometallic catalysts (Fig. 1b) are appealing because they enable inner-sphere catalysis where the reactants form a chemical bond with the mediator (e.g., to the metal atom center), stabilizing intermediates and reducing energy barriers (Fig. 1c). Like homogeneous catalysts in thermochemical reactions, however, homogeneous electrochemical redox mediators must also be separated and recovered after the reaction, and scalable continuous processes are difficult to design. Additionally, the thermodynamics and kinetics of a reaction in a system with a homogeneous catalyst are affected by temperature and pressure because they are bulk properties. In an electrochemical redox-mediated system, the electrochemical driving force is decoupled from the reaction center on the mediator, and the reaction chemistry is controlled primarily by the mediator. Such systems are limited by the concentration of the mediator and cannot take advantage of the scalable surface area of a heterogeneous catalyst (Fig. 1b). For these reasons, electrochemical systems where energy efficiency and cost are major driving factors often use inner-sphere heterogeneous catalysts, such as for water splitting, carbon dioxide
reduction, and fuel cells.13–15 Catalysts in these systems range from single atoms of platinum-group metals to monolayers of transition metal dichalcogenides, building on decades of development in materials synthesis to optimize the reaction energetics.

Heterogeneous Catalysts for Future Electrochemical Syntheses
Despite the wide success in applying cheap, energy-efficient catalysts to a few specific electrochemical reactions, electrochemical syntheses involving larger molecules and more complex functional groups generally rely on redox mediators. Recent research has successfully begun developing inner-sphere routes on heterogeneous electrodes for reactions, including carbonyl reductions,16,17 oxygenatom transfers,18 carbon-nitrogen bond formations,19–21 and more (Fig. 1d). In these cases, a range of transition metals, metal oxides, and metal nanoparticles are used as heterogeneous electrocatalysts; yet these examples are still limited compared to the extensive library of catalysts used in water electrolyzers or thermochemical reactors. Developments in thermochemical systems, such as precise atomic control of catalysts,22,23 can be leveraged to enable scalable, energy-efficient, and selective electrochemical transformations. Such heterogeneous electrocatalytic systems will help decarbonize electrochemical synthesis while also enabling new paradigms in chemical manufacturing that take advantage of the new synthesis routes and the decentralized nature of electrochemical systems.
38 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org Schiffer (continued from previous page)
Fig. 1. (A) United States chemical manufacturing sector energy usage by subsector. Basic organic chemicals use 20% of the energy demand of the sector. Data from Ref 4. (B) Schematic of redox-mediated electrochemical synthesis for the reaction A→B. Note that the influence of the applied potential primarily occurs near the electrode surface and does not directly influence the desired chemical reaction. (C) Schematic comparison of inner-sphere and outer-sphere catalysis for the example case of ammonia electro-oxidation. In general, inner-sphere reactions are less energy intensive because intermediates are stabilized by bonds with the catalyst.
Acknowledgment
The author is funded by the Liquid Sunlight Alliance, which is supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, Fuels from Sunlight Hub under Award Number DE-SC0021266 and the US Department of Energy, Advanced Research Projects Agency–Energy (ARPA-e) under contract number DE-AR0001407. The author would also like to acknowledge The Resnick Sustainability Institute at Caltech. Additionally, the author would like to thank Nicholas Watkins and Karthish Manthiram for discussions and feedback on this work.
© The Electrochemical Society. DOI: 10.1149/2.F05231IF
About the Author
Zachary Schiffer, Resnick Sustainability Postdoctoral Scholar, Applied Physics & Materials Science, Caltech

Education: BSE in Chemical and Biological Engineering (Princeton University) with senior thesis work on the mechanics of Li-ion batteries with Prof. Craig Arnold; PhD in Chemical Engineering (MIT) under Prof. Karthish Manthiram.
Research Interests: Postdoctoral scholar with Prof. Harry Atwater at Caltech, where his research focuses on electrochemical carbon capture from seawater and photocatalytic nitrogen reduction. His graduate thesis work focused broadly on exploring electrification and decarbonization routes for industrial chemical processes, with a focus on the development of electrochemical routes for ambient-condition nitrogen cycle reactions. In general, his research combines fundamental thermodynamics, kinetic analysis techniques, computational chemistry, and materials synthesis to explore electrochemical systems.
Honors & Awards: 2022 ECS Energy Technology Division Graduate Student Award Sponsored by BioLogic. https://orcid.org/0000-0001-6069-8613
About the Editor
Scott Cushing, Assistant Professor of Chemistry, Caltech

Education: BS in Physics, emphasis in Material Science and Chemistry and PhD in Physics, under Nick Wu and Alan Bristow (West Virginia University).
Research Interests: With a multidisciplinary background spanning Chemistry, Materials Science, and Physics, his research focuses on the creation of new scientific instrumentation that can translate quantum phenomena to practical devices and applications. The Cushing lab is currently pioneering the use of attosecond x-ray, time-resolved TEM-EELS, and ultrafast beams of entangled photons for a range of microscopy and spectroscopy applications.
Work Experience: Past appointments include Dept. of Energy EERE Postdoctoral Fellow, Prof. Stephen Leone Group University of California, Berkeley with a Co-Appointment at Lawrence Berkeley National Laboratory. Currently Senior Research Advisor for Pacific Integrated (PI) Energy, San Diego, CA.
Pubs & Patents: >60 publications, 3 patents, h-index >30, cited ~8,000 times
Awards: 2022 Cottrell Scholar, 2022 Shirley Malcom Prize for Excellence in Mentoring, 2019–2021 Young Investigator awards for DOE, AFOSR, ACS, and Rose Hill Foundation.
Work with ECS: ETD Division: assist with organizing and chairing symposium. Member for >15 years.
Website: cushinglab.caltech.edu
https://orcid.org/0000-0003-3538-2259
References
1. IEA, ICCA, and DECHEMA. Technology Roadmap: Energy and GHG Reductions in the Chemical Industry via Catalytic Processes (2013).
2. Z. J. Schiffer and K. Manthiram, Joule, 1, 10 (2017).
3. E. J. Biddinger and M. A. Modestino, ECS Interface, 29 (3), 43 (2020).
4. US Department of Energy, Bandwidth Study on Energy Use and Potential Energy Saving Opportunities in US Chemical Manufacturing (2015).
5. G. G. Botte, ECS Interface, 23 (3), 49 (2014).
6. Z. J. Schiffer, A. M. Limaye, and K. Manthiram, Joule, 5 (1), 135 (2021).
7. I. Chorkendorff and J. W. Niemantsverdriet, Concepts of Modern Catalysis and Kinetics, Wiley Interscience, New York (2003).
8. R. A. Sheldon and H. van Bekkum, Fine Chemicals through Heterogeneous Catalysis, Wiley Interscience, New York (2007)
9. C. Zhu, N. W. J. Ang, T. H. Meyer, Y. Qiu, and L. Ackermann, ACS Cent Sci, 7, 7 (3), 415 (2021).
10. Y. Yuan and A. Lei, Nat Commun, 11, 802 (2020).
11. D. Pollok and S. R. Waldvogel, Chem Sci, 11, 12386 (2020)
12. M. Yan, Y. Kawamata, and P. S. Baran, Chem Rev, 117 (21), 13230 (2017).
13. S. Nitopi, E. Bertheussen, S. B. Scott, et al., Chem Rev, 119 (12), 7610 (2019).
14. S. Anantharaj, S. R. Ede, K. Sakthikumar, K. Karthick, S. Mishra, S. Kundu, ACS Catal, 6 (12), 8069 (2016).
15. Q. Fu, J. Han, X. Wang, P. Xu, et al., Adv Mat (2021).
16. A. S. May and E. J. Biddinger, ACS Catal, 10 (5), 3212 (2020).
17. J. A. Lopez-Ruiz, E. Andrews, S. A. Akhade, et al., ACS Catal, 9 (11), 9964 (2019).
18. K. Jin, J. H. Maalouf, N. Lazouski, N. Corbin, and D. Yang, J Am Chem Soc 141, 6413 (2019).
19. R. Xia, D. Tian, S. Kattel, B. Hasa, H. Shin, X. Ma, J. G. Chen, and F. Jiao, Nat Commun 12 (1), 1 (2021).
20. Z. J. Schiffer, M. Chung, K. Steinberg, and K. Manthiram, Submitted 2022.
21. M. Jouny, J. J. Lv, T. Cheng, B. H. Ko, J. J. Zhu, W. A. Goddard, and F. Jiao, Nat Chem 11, (9), 846 (2019).
22. R. Jin, G. Li, S. Sharma, Y. Li, and X. Du, Chem Rev, 121 (2), 567 (2021).
23. L. Liu and A. Corma, Chem Rev, 118 (10), 4981 (2018).
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 39
Electrochemistry in Action:
edited by Christopher L. Alexander, Interface Contributing Editor
Engineering the Neuronal Response to Electrical Microstimulation
 by M. E. Orazem and Kevin J. Otto
by M. E. Orazem and Kevin J. Otto
Brain neuromodulation has revolutionized the medical treatment of neurological diseases and injuries that include Parkinson’s disease, essential tremor, epilepsy, paralysis, stroke, depression, and Tourette’s syndrome; however, existing therapies are limited in their clinical scope of application. Most existing therapies are delivered through implanted macroelectrodes (mm2 surface area) that reside either on top of or directly inside the brain. Estimates of the effective electric field spread from these devices generally span from thousands to millions of individual neurons.1–3 Unfortunately, some neurological diseases and injuries require stimulation fields of higher precision. Next-generation microneuromodulation devices (~102 –103 µm2 surface area) have been developed by others with hundreds of closely spaced channels. 4–6 These devices may be able to provide electrical microstimulation in the form of biphasic, charge-balanced small amplitude square waves that provides salient, behaviorally relevant information to human subjects. However, there is a lack of knowledge incorporated into their safety and clinical usage (e.g., spatiotemporal patterning of stimulation).

Neuromodulation is defined by the International Neuromodulation Society (INS) as a field of science, medicine, and bioengineering that encompasses implantable and non-implantable technologies, electrical or chemical, that act upon neural interfaces to improve life for humanity.7 Our research groups collaboratively investigate neuromodulation performed via electrical microstimulation (Fig. 1). Our primary development target is brain neuromodulation. In this article we highlight the application of electrochemistry to the field of neuromodulation.
In a new multi-investigator NIH grant entitled “Engineering the Neuronal Response to Electrical Microstimulation,” we seek to characterize, model, and validate the membrane, cellular, extracellular, circuit, and adaptive-biological responses of the brain cells (including neuronal and non-neuronal cells) to electrical microstimulation. The overall objective of our work is to identify the spatial, temporal, and spatiotemporal resolution of a chronically implanted electrical microstimulation device. We will accomplish our objective through a combined approach employing electrochemical impedance modeling, biophysical modeling, electrophysiology, and psychophysics. Our central hypothesis is that a fundamental understanding of the charge transfer and impedance response of the electrode-tissue interface will enhance the rate and fidelity of information transfer and establish a theoretical framework for rational electrode design. We are joined in this effort by Stuart Cogan, University of Texas at Dallas; Warren Grill, Duke University; and Daryl Kipke, NeuroNexus.
To approach the electrochemical aspect of our objective, we seek to develop impedance models of non-linear, dynamic microneuromodulation systems. We will apply electrochemical impedance spectroscopy (EIS) approaches in device evaluation, including: 1) measurement modeling and 2) process modeling of impedance measurements from microneuromodulation devices, 3) finite element model (FEM) simulations of impedance, and 4) FEM simulations of current flows during stimulation and the transient electrochemical response.
Our measurement model provides powerful pre-processing of EIS data, quantifying noise and identifying inconsistencies with the Kramers−Kronig relations. By quantifying the error structure of EIS measurements8 and filtering lack of replication of similar impedance spectra, the measurement model can be used to identify the stochastic error of impedance measurements.9
The measurement model can also identify the frequency range of EIS measurements that are consistent with the Kramers–Kronig relations, implying measurement stationarity.10
Knowledge of the error structure of the data is essential for interpretation.11
The inverse of the variance for the stochastic error structure is used to weight regressions, and the weighted chi-square statistic is used as a means of determining quality of fit. Only the part of the spectrum that is consistent with the Kramers–Kronig relations is
40 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org
Fig. 1. Electrochemical neuromodulation: (a) schematic representation of an array of microelectrodes (vertical insulated cylinders with the end exposed). The neurons are stimulated by an electric field that extends a small distance from the electrode at the end of the insulated cylinder, represented by a blue spherical cloud. (b) insertion of an electrode array into the brain. Images taken from the Otto group.
used for regression of the interpretation model, thus eliminating the confounding influence of nonstationary phenomena and instrument artifacts. In recent work, we have shown that the measurement model can provide an estimate of capacitance,12 allowing identification of the frequency above which the ohmic impedance associated with the electrode geometry can confound interpretation.13 Our group recently published our program.14 In the proposed efforts we will apply the measurement model to new EIS data as well as teach its use to the broader neuroscience community.
Our team has emphasized process model interpretation of EIS data in terms of proposed physics and reaction mechanisms. The powerlaw model, for example, allowed interpretation of the impedance response of oxide films in terms of film thickness.15 Mechanistic models were used to quantify the corrosion of cast iron pipes used in France for distribution of potable water.16 Mechanistic models were also used to show that the oxidation of the platinum catalyst can cause low-frequency inductive loops in the impedance response of PEM fuel cells.17,18 Our measurement model program allows regression of custom models to EIS data, and we will apply this approach to generate equivalent circuit interpretation models of EIS behavior.
FEM models accounting for the nonlinear transient behavior of electrochemical systems can provide critical understanding of microneuromodulation stimulation. These models should account for the steady-state impedance,19 and the transient response20 of the electrode-electrolyte interface. Prior work shows that geometryinduced nonuniform current and potential distributions21 influence the impedance response, yielding, at sufficiently high frequencies, a frequency dispersion22 that we have termed an ohmic impedance 13 The frequency at which the ohmic impedance is observed is increased by a reduction of electrode size, thus expanding the useful frequency range.23 In preliminary work, we found that potential transients cause temporal changes in the current and potential distribution that are affected by ReC and RtC time constants. Temporal changes in ohmic resistance agreed with the ohmic impedance calculations. Current transients, however, are influenced only by the RtC time constant. The resulting potential excursion, however, is strongly dependent on the contributions of both capacitive and faradaic currents.
The formal analysis of the electrochemical behavior of electrodes, both in vivo and in vitro, will guide development of the FEM models for stimulation pulses. Our work will further advance research by generating software and hardware technology that will be used widely throughout the research community. Furthermore, our efforts are translatable to clinical practice. We hope that our efforts will help to reduce the burden of human disability through disease or injury. © The Electrochemical Society. DOI:10.1149/2.F06231IF
About the Authors
Mark E. Orazem, Distinguished Professor of Chemical Engineering, University of Florida
Education: BS and MS (Kansas State University) and PhD (University of California, Berkeley) in Chemical Engineering.
Research Interests: Electrochemical engineering, electrochemical impedance spectroscopy, corrosion (including cathodic protection), current distribution in electrochemical systems, fuel cells, batteries, mathematical modeling.
Work Experience: 1983-1988 Department of Chemical Engineering, University of Virginia; 1988-present Department of Chemical Engineering, University of Florida
Honors & Awards: ECS Corrosion Division H. H. Uhlig Award (2022); Herbert Wertheim College of Engineering Doctoral Dissertation Adviser/Mentoring Award (2021); Fellow of the International Society of Electrochemistry; Inaugural Triennial Claude Gabrielli Award (2019);
ECS Henry B. Linford Award for Distinguished Teaching (2012); Past President of the International Society of Electrochemistry; Fellow of the Electrochemical Society.
Pubs + Patents: >210 refereed publications and co-author, with Bernard Tribollet of the CNRS in Paris, of Electrochemical Impedance Spectroscopy and editor of Underground Pipeline Corrosion Website: https://www.che.ufl.edu/orazem/

Kevin J. Otto, Professor and Senior Associate Chair, J. Crayton Pruitt Family Department of Biomedical Engineering, University of Florida

Education: BS in Chemical Engineering (Colorado State University), MS and PhD in Bioengineering (Arizona State University) Research Interests: Engineering neural interfaces for both research purposes as well as treatment options in neurological injuries or disease. In particular, his research focuses on multi-channel implantable microdevices in both the central and peripheral nervous systems. These interfaces are being investigated for many applications, including: sensory replacement, cognitive functional therapy, and neuromodulation for autonomic therapies.
Work Experience: Post-doctoral training at University of Michigan; appointments in the Departments of Neuroscience, Neurology, Materials Science & Engineering, and Electrical & Computer Engineering, University of Florida
Pubs + Patents: h-index: 31; i10 index: 64; >123 peer-reviewed publications
Honors & Awards: Fellow, Biomedical Engineering Society; Fellow, American Institute for Medical and Biological Engineering; National Biomedical Engineering Society Annual Conference CoChair (2017)
Website: https://nprlab.org/ https://orcid.org/0000-0002-2317-6194
About the Editor
Christopher L. Alexander, Assistant Professor of Civil & Environmental Engineering and Susan and William Bracken Junior Faculty Fellow, University of South Florida
Education: BS and ME in Civil Engineering and PhD in Chemical Engineering (University of Florida).
Research Interests: Directs the corrosion research laboratory, which aspires to conquer corrosion while increasing the sustainability and resilience of critical infrastructure. Current projects include the corrosion performance of anodized aluminum formed with novel additives, susceptibility of prestressing steels placed within galvanized steel ducts to hydrogen embrittlement, reinforcement corrosion control for sustainable concrete formulations, corrosion propagation mechanisms of stainless steel concrete reinforcement, the role of coating deficiencies on the service life of metallic coated drainage pipes, and corrosion degradation of wastewater treatment plant components.
Work Experience: Postdoctoral fellow at Sandia National Laboratories within the Materials Reliability Center where he studied atmospheric stress corrosion cracking as it relates to the aging and lifetime of nuclear waste interim storage containers. Honors & Awards: McKnight Dissertation Fellowship (2016–2017), DOW BEST Symposium (2016), University of Michigan NextProf (2016).

Website: http://cee.eng.usf.edu/faculty/clalexa2/
(continued on next page)
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 41
Orazem and Otto
(continued from previous page)
References
1. C. C. McIntyre, S. Mori, D. L. Sherman, N. V. Thakor, and J. L. Vitek, Clin. Neurophysiol., 115 (3), 589 (2004).
2. L. Liu, J. Zhang, J. Sun, and K. Xu, Biomed. Eng., 21 (1), 58 (2022).

3. N. Yousif, and X. Liu, Expert Rev. Med. Devices, 4 (5), 623 (2007).
4. G. Guitchounts, J. E. Markowitz, W. A. Liberti, and T. J. Gardner, J. Neural Eng., 10 (4), 046016 (2013).

5. L. Luan, X. Wei, Z. Zhao, et al., Sci. Adv. 3 (2), 1 (2017).
6. J. J. Jun, N. A. Steinmetz, J. H. Siegle, et al., Nature, 551 (7679), 232 (2017)
7. R. Davis, P. L. Gildenberg, G. Barolat, et al., in Neuromodulation, E. S. Krames, P. H. Peckham, and A. R. Rezai, Editors, p. 49, Academic Press, San Diego (2009)
8. P. Agarwal, M. E. Orazem, and L. H. Garcia‐Rubio, J. Electrochem. Soc., 139, 1917 (1992)
9. P. Agarwal, O. D. Crisalle, M. E. Orazem, and L. H. Garcia‐Rubio, J. Electrochem. Soc., 142 (12), 4159 (1995)
10. P. Agarwal, O. D. Crisalle, M. E. Orazem, and L. H. Garcia‐Rubio, L. H., J. Electrochem. Soc., 142 (12), 4149 (1995)
11. M. E. Orazem, P. Agarwal, A. N. Jansen, P. T. Wojcik, and L. H. Garcia-Rubio, Electrochimica Acta, 38, 1903 (1993).
12. H. Liao, W. Watson, A. Dizon, et al., Electrochimica Acta, 354, 136747 (2020).
13. O. Gharbi, A. Dizon, M. E. Orazem, et al., Electrochimica Acta, 320, 134609 (2019)
14. W. Watson and M. E. Orazem, EIS: Measurement Model Program, 2020.
15. M. E. Orazem, I. Frateur, B. Tribollet, et al., J. Electrochem. Soc., 160, C215 (2013)
16. I. Frateur, C. Deslouis, M. E. Orazem, and B. Tribollet, Electrochimica Acta, 44 (24), 4345 (1999)
17. S. K. Roy, M. E. Orazem, and B. Tribollet, J. Electrochem. Soc., 154, B1378 (2007)
18. S. K. Roy, H. Hagelin-Weaver, and M. E. Orazem, J. Power Sources, 196 (8), 3736 (2011)
19. A. Dizon and M. E. Orazem, Electrochimica Acta, 327, 135000 (2019)
20. Y.-C. Chang, R. Woollam, and M. E. Orazem, J. Electrochem. Soc., 161 (6), C321 (2014)
21. J. Newman, J. Electrochem. Soc., 113, 1235 (1966)
22. J. Newman, J. Electrochem. Soc., 117, 198 (1970).
23. V. M.-W. Huang, V. Vivier, M. E. Orazem, et al., J. Electrochem. Soc., 154, S8 (2007)
42 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org
www.electrochem.org/PRiME2024 PRiME 2024 JOINT INTERNATIONAL MEETING of The Electrochemical Society of Japan, The Korean Electrochemical Society, and The Electrochemical Society SAVE THE DATE HONOLULU, HI October 6-11, 2024 Hawaii Convention Center & Hilton Hawaiian Village
Corrosion Behavior of Cu in Accelerated Hydrogen Peroxide–Based Disinfectants
Cu is widely recognized as a self-sanitizing material to deactivate or kill pathogenic bacteria, thanks to the release of Cu ion during the aqueous corrosion process. A study conducted by Nakhaie et al. investigated the corrosion mechanism of Cu when exposed to commercially available AHP® (accelerated hydrogen peroxide) disinfectants. ViroxTM/MC and Accel®, which both contain phosphoric acid and other chemicals in addition to H2O2, were chosen for the study. Cu coupons were immersed in Virox, Accel, and H2O2-only solutions for 7 days followed by SEM and XPS characterizations. The corrosion rate during the 7-day immersion followed this order: Virox > Accel > H2O2. The corrosion rates of both Virox and Accel decreased over time due to formation of corrosion product on the surface. XPS analysis showed that chelating agents such as PO43− from both Virox and Accel accelerated Cu corrosion in a H2O2 solution since the formed complexes did not passivate the surface. The presence of another chelating agent for Cu in Virox, hydroxyethylidene diphosphonic acid (HEDP), contributed to its higher corrosion rate than that of Accel. Whenever AHP disinfectants are used, the minimum amount of Cu ion released to kill bacteria must be weighed against accelerated corrosion.
From: D. Nakhaie, A. M. Clifford, and E. Asselin, J. Electrochem. Soc., 169, 101504 (2022)
Hydrogen Crossover in PEM Water Electrolysis at Current Densities up to 10 A cm−2 Understanding and mitigating hydrogen crossover in PEM water electrolyzers is critical for improved safety and efficiency. Researchers from Leibniz University in Hanover, Germany investigated the unresolved functional relationship of hydrogen crossover with current density. Analyzing crossover performance at both ambient and elevated pressures, the team classified their observations into three regions as a function of current density, with the hydrogen crossover flux being linear in the first region, followed by a nonlinear region in the second, and a flattening behavior in the third. The team attributed the first region to increased partial pressure of hydrogen on the cathode side, the second region to mass transport effects on the cathode side, and the third region to an additional effect of hydrogen dissolved in water being transported back from the anode to cathode through electro-osmotic drag. This was corroborated with the movement of the initiation of the flattening Region 3 to lower current densities at higher pressures. The researchers concluded that one should consider both the diffusive and the electro-osmotic drag of hydrogen to truly
understand and mitigate hydrogen crossover as a function of operating current density.
From: A. Martin, P. Trinke, et al., J. Electrochem. Soc., 169, 094507 (2022)
A Surface Modification Strategy Towards Reversible Na-ion Intercalation on Graphitic Carbon Using Fluorinated Few-Layer Graphene
The Na-ion battery (NIB) has been receiving ever increasing attention due to the lithium-ion battery’s supply chain issues and soaring raw-material price. One of NIB’s challenges is on its anode side, where only hard, non-graphitizable carbons can be used to achieve reversible intercalation with low capacity and varying performance depending upon the carbon source and production method. In a recent report, researchers from the University of Illinois at Urbana-Champaign, the Michigan State University, and the Florida State University investigated the use of graphene as anode materials for NIBs. The authors have previously performed theoretical simulations showing the thermodynamically favorable intercalation of Na+ into graphene structures with fluorine surface modification (ACS Appl. Mater. Interfaces, 12, 19393 (2020)). In their current work, experimental results from ion-sensitive SECM, in situ Raman spectroscopy, and electrochemical measurements were presented to confirm the formation of a Stage-3 type intercalation structure with stoichiometries around NaC14–18, which is significantly higher than the previously reported NaC186 from graphite. In addition, ex-situ XPS characterizations revealed the necessity of a preformed Libased solid electrolyte interface layer to realize reversible Na+ intercalation with the fluorinated graphene structures.
From: D. Sarbapalli, Y-H. Lin, S. Stafford, et al., J. Electrochem. Soc., 169, 106522 (2022).
Fiber Optic Based Thermal Sensing of Lithium-Ion Cells at the Module Level Although lithium-ion batteries are widely used, the ability to monitor, detect, and predict potential safety issues, such as thermal runaway, is an area of active research. Battery management systems (BMS) have been developed to provide an early indication of these safety concerns through the monitoring of individual cell voltages and a few temperatures across a module. In this paper, researchers at the University of Texas at Arlington, Luna, and the Naval Surface Warfare Center investigated whether a high-definition fiber optic sensor (HD-FOS), purchased commercially, could be integrated within a module to provide discrete temperature measurements across individual cells. Their results demonstrate the integration and use of HD-FOS to sense individual cell temperatures under a variety of conditions,
such as normal, overcharged, and shortcircuit. The ability to sense individual cell temperatures would greatly refine the diagnostic and prognostic capabilities of the BMS, by pinpointing exactly the source of a temperature increase within a module. While not shown here, it is thought that the same sensing technique could be used for measuring strain, another potential safety indicator. Both measurements could allow preventative measures to be initiated immediately, potentially halting a catastrophic failure.
From: H. Atchison, Z. Bailey, D. Wetz, et al., J. Electrochem. Soc., 169 097503 (2022)
Electrochemical Performance of Fe2O3@PPy Nanocomposite as an Effective Electrode Material for Supercapacitor Supercapacitors remain one of the most important energy storage systems, alongside rechargeable batteries and fuel cells. However, issues such as relatively low energy densities compared to lithium-ion batteries continue to be a challenge for the development of practical supercapacitors. Consequently, there is a significant research effort to identify promising supercapacitor electrode materials to increase electrochemical performance. To this end, researchers from Periyar University and Vidyaa Vikas College of Arts and Science have recently reported on the application of a composite of iron oxide and polypyrrole (Fe2O3@PPy) as a supercapacitor electrode material. The composite was prepared via facile mixing at low temperature. A comparison of results from galvanostatic cycling revealed that the Fe2O3@PPy composite offers significantly increased charge stored compared to PPy and Fe2O3 samples cycled on their own. Cyclic voltammetry demonstrated a capacity of 395.45 C g−1 at a scan rate of 5 mV s−1. This work demonstrates that the electrochemical performance of metal oxides, which are used as supercapacitor electrode materials, may be enhanced through the preparation of composites with conductive polymers.
From: N. Balasubramanian, S. Prabhu, N. Sakthivel, et al., J. Solid State Sci. Technol., 11, 091001 (2022)
Tech Highlights was prepared by Mara Schindelholz of Sandia National Laboratories, David McNulty of University of Limerick, Chao (Gilbert) Liu of Shell, Zenghe Liu of Abbott Diabetes Care, Chock Karuppaiah of Vetri Labs, and Donald Pile of EnPower, Inc. Each article highlighted here is available free online. Click on the article reference to take you to the full-text version of the article.
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 43 TECH HIGHLIGHTS TECH HIGHLIGHTS
ECS News
WHY READ ECS NEWS?
by Frances Chaves
ECS News: your connection to the ECS community, career opportunities, and industry-related news and events.
Publishing is the bedrock of a career in science
Learn about upcoming ECS journal focus issues, topics, calls for papers—and meet important deadlines.
Scientific prizes confer recognition and credibility

Read about upcoming Society, division, and section prizes and awards, including deadlines, nomination requirements, and who is winning what and why!
Science isn’t static. Accelerate your knowledge
Retraining and upskilling keep you on top of your field. Educate yourself about upcoming ECS continuing education opportunities, including webinars, Short Courses, and professional development opportunities.

1 2 3 4 5 6
What you knew about job searching yesterday may not be true today

Posts from the ECS Career Center provide up-to-date information on job search trends. Be ready to present the best you in your job search.
Your research deserves an audience— and so do you
ECS biannual meetings are THE place to present research in electrochemistry and solid state science to THE audience that matters the most. Review meeting topic close-ups: indepth descriptions of what symposia organizers are looking to present.
ECS is a member-driven association
As a member, you have a voice in determining the Society’s leadership. Learn about candidates for volunteer positions—and volunteer opportunities that may benefit you.
www.electrochem.org/ecsnews

44 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org
Special Issue of Interface on Neuromorphic Computing: An Introduction and State of the Field
by Durgamadhab Misra, Special Issue Guest Editor
The human brain integrates and processes information to perform complex cognitive tasks within approximately 20 watts of power. Today’s fastest supercomputer is unable to deliver the power requirements and the number of operations at the same energy levels. In the brain, the discrete and sparse events in time called spikes are used to process and encode the information. The energy efficiency of the brain is attributed to the sparsity of the spikes and event-driven communication between the neurons. Complex interconnections among the 1011 neurons and 1015 synapses in the human brain process the information, possibly encoded in the time, frequency, and phase of the spikes. Therefore, to emulate human cognition requires novel electronic devices and new algorithmic approaches. Brain-inspired computing, or neuromorphic computing, is an approach to build energy-efficient computing architectures and systems.
Our understanding of the brain thus far indicates that synapses that connect two neurons serve as memory elements and that the neurons act as computational nodes. Learning is typically achieved by modulating the synaptic strength between the neurons, which is termed synaptic plasticity. Memory and computational elements, which are physically separated in modern digital computers (called Von Neumann computers), are not energy-efficient in handling dataintensive workloads. On the other hand, in a neuromorphic computer, both processing element and memory, governed by the neurons and the synapses, are co-located, which allows some computing to be done in the memory element. Data-intensive machine learning and artificial intelligence implementations are more suitably implemented by neuromorphic computing.
A lot of efforts are on-going to demonstrate brain-like energy efficiency in silicon-based computing systems using existing and evolving devices and circuits. Many challenges associated with in-memory computing and with implementing large-scale neural networks in silicon are yet to be resolved. In this context, Prof. Rajendran, in his article, “Building a Smart and Green AI,” suggests that there is a requirement for developing novel devices using novel nanoscale solid state and electrochemical materials which will be key enablers for next-generation AI technologies. These nanoscale devices will be potential candidates to emulate the brain’s connectivity in hardware owing to their small size and low programming energy requirements.

So, what we are looking for is an implementation of electronic synapses and neuron circuits using silicon technology. Many papers are being published in the ECS Journal of Solid-State Science and Technology and presented at the ECS meetings on nanoscale resistive switching devices for neuromorphic applications to replicate the synapses in the brain. The resistive switching device, or the electronic synapse, is based on conductance variation across a two-terminal device (metal-insulator-metal [MIM]) to an applied electric potential and subsequently returning to its original state when a reverse potential is applied. It is also referred to as a resistive random-access memory (ReRAM or RRAM) device. Professor Jha in her article “Emerging
Memory Devices Beyond Conventional Data Storage: Paving the Path for Energy-Efficient Brain-Inspired Computing” has outlined the detailed operation of the device and its circuit configuration to implement neuromorphic architectures.
One of the materials used in electronic synaptic devices is phasechange material (PCM), which depends on an amorphization and crystallization process for the change in conductance. The ferroelectric dielectric material is used as a switching layer that changes the polarization and behaves like a synapse. Copper has been introduced in silicon dioxide to make a switching layer where the conductance is modulated by mobile ions. When oxygen vacancies in the dielectric are used for the conductance modulation in the switching layer, transition metal oxides like amorphous HfO2based dielectric are considered. However, low oxygen vacancies in stoichiometric HfO2 limit the low power switching operation. Therefore, HfOx (with x < 2) has been used as the switching layer. Since HfO2 is well integrated into silicon technology, today many dielectrics and metal stacks are specifically engineered to enhance power efficiency and reduce performance variations in the switching layer. This includes bilayer dielectrics (Al2O3 – HfO2) and plasma treatment during HfO2 deposition. While all the switching devices demonstrate excellent conductance quantization to a varying electrical pulse and reduced switching energy, they still exhibit some level of device-to-device or in-device stochasticity. The main challenge is, therefore, to identify appropriate materials for the switching layer and novel architectures for the successful implementation of an electronic synapse for neuromorphic computing.
© The Electrochemical Society. DOI: 10.1149/2.F08231IF
About the Author
Durga Misra, Professor and Department Chair, Electrical and Computer Engineering, New Jersey Institute of Technology
Education: MS and PhD in Electrical Engineering (University of Waterloo, Canada). Research Interests: Has been working on nanoscale semiconductor devices, especially on gate dielectrics especially in high-k gate stack engineering for nanoscale devices and device reliability for the last 35 years. His current research interest is in the design and processing of low power devices using high-k dielectrics for in-memory computing.
Honors & Awards: Include Fellow of ECS and Awarded Life Member. Recipient of the ECS Tomas Callinan Award and the Electronic and Photonic Division Award. Fellow of IEEE.
Work with ECS: He has been the Chair of Dielectric Science and Technology Division of ECS and has been organizing technical symposia at ECS for the last 30 years at ECS.
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 45
“A lot of efforts are on-going to demonstrate brain-like energy efficiency in siliconbased computing systems using existing and evolving devices and circuits.”
SAVE 20%
The Electrochemical Society book series provides authoritative, detailed accounts on specific topics in electrochemistry and solid state science and technology. These titles are sponsored by ECS and published in cooperation with Wiley.






SECOND EDITION
ATMOSPHERIC CORROSION


LaQue’s Handbook on Marine Corrosion, 2nd Edition


David A. Shifler

ISBN 978-1-119-78883-6 | July 2022


EDITED BY DAVID A. SHIFLER LAQUE’S HANDBOOK OF MARINE CORROSION THE ELECTROCHEMICAL SOCIETY SERIES SECOND EDITION
Shop the Latest ECS Titles at wiley.com
INTERESTED IN PUBLISHING WITH US?
Please contact publications@electrochem.org to discuss your idea.

- CD5827
ON THE MOST TRUSTED RESOURCES IN ELECTROCHEMISTRY New
A practical, single-source reference on the unique nature of seawater as a corrosive environment. Explains practical corrosion control solutions via design, proper materials selection, and implementation of good corrosion control engineering practices.
Christofer Leygraf Inger Odnevall Wallinder Johan Tidblad Thomas Graedel
ECS members receive 20% off all Wiley books.
ECS members use promo code ECS18 at checkout.
Building a Smart and Green AI

 by Bipin Rajendran
by Bipin Rajendran
The nature of computing has undergone a fundamental paradigm shift over the past two decades – as opposed to compute-intensive workloads of the last century, more and more application domains are now data-intensive.1 Some notable examples include data search and mining, analyses of social networks and sensor networks, and environmental and economic modeling as well as the development of artificial intelligence (AI) models that are now being deployed in many of these application domains. The global datasphere is projected to grow 4-fold to 175 zettabytes (or 175 billion terabytes) over the next 5 years; at the same time, the volume of data that needs to be processed using “cognitive systems” is expected to grow by a factor of 100.2
Over the past decade, thanks to fundamental advances in machine learning (ML) based on artificial neural networks (ANNs), AI is quickly becoming a general-purpose technology with potential transformative impact similar to that of electricity and information and communications technologies (ICT) in the past two centuries. AI models based on deep learning have demonstrated super-human performance for a wide variety of tasks—ranging from language translation3 to protein folding.4
AI research today focuses primarily on improving the overall accuracy of predictions that require huge amounts of well-annotated data. To train these state-of-the-art AI models with ever-increasing complexity, increasing amounts of computational resources are needed. Over the past decade, the computational resources required to develop and deploy AI models have sky-rocketed,5,6 and for some state-of-the-art deep–learning models, the energy required for this can be as high as the equivalent of driving a car to the moon and back.7

These trends pose a significant challenge for the computing industry: How do we design cognitive computing systems that not only deliver well-calibrated and trustworthy decisions, but can also be deployed in the field, are capable of adapting or learning the features of unseen input streams in a secure manner, and operate within the energy budgets of battery-operated devices?
Evolution has perfected a computational engine—the human brain—that may offer some clues to this problem. Even though
it too operates with imperfect data and an inherently stochastic computational fabric made of biological neurons and synapses, our brains continuously make complex decisions while requiring only about 20 Watts. Why is human-engineered AI technology lagging so drastically behind the capabilities and efficiency of the human brain? There may be three fundamental reasons for this: (i) Deep neural networks or DNNs encode and process information using high-precision real numbers, completely ignoring the notion of time. On the other hand, the brain utilizes sparse, temporal, binary signals called spikes to encode, transmit, and process information. (ii) Computing systems today employ the von Neumann architecture where data is processed in units that are physically separated from memory blocks that store data. For data-intensive computations, this physical separation becomes a bottleneck,8 as data must be shuttled back and forth through relatively long communication buses. The brain, on the other hand, employs a dense 3-D connected network of compute and storage nodes— the neurons and synapses— thereby closely integrating processing and memory. (iii) The fundamental building blocks of today’s Si computers—the CMOS transistors—are leaky switches that operate at relatively high voltages and currents compared to those of the neurons and synapses in the brain. The sub-100mV spike signals that transmit information in the brain, as well as nanoAmpere scale currents that are used in signal integration, provide an ultra-low-power substrate to realize complex computing functions.
Hence, to realize the vision of a smart and green AI, we need to develop principled algorithms, nanoscale devices, and architectural paradigms that are inspired by the key organizational features of the brain. This is a paradigm shift from the “Red AI”9 mode of research pursued today that features exponentially increasing model complexity and computing costs to obtain, at best, linear improvements in the accuracy of decision-making. The Red AI approach is so prevalent today primarily because AI research largely remains siloed—on the one hand, software research focuses on discovering and improving the accuracy of AI algorithms without optimizing for computational efficiency. In contrast, hardware research aims to faithfully implement the algorithms in custom hardware, which often results in substantial computational costs. To develop ubiquitously deployable AI that can generate actionable intelligence in a local and distributed manner, the design of AI algorithms and hardware accelerators must go hand-inhand—targeting both fundamental advances and co-optimization of algorithms and hardware technologies.
There are several plausible approaches to this. We can draw inspiration from the human brain’s event-driven, probabilistic computational features, and develop algorithms that naturally use the temporal dimension for information encoding and processing, using sparse binary signaling schemes and event-driven local plasticity rules. As opposed to the commonly used frequentist model of AI that aims to generate point estimates for model parameters that can only provide decisions or recommendations, models based on Bayesian principles can provide quantifiable estimates of confidence or uncertainty, which are crucial to making AI trustworthy. In this regard, the Free Energy Principle (FEP) proposed by Friston10 holds great promise, and can also be used to develop a unified approach to AI algorithms that are hardware friendly. According to the free energy (continued on next page)
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 47
“How do we design cognitive computing systems that not only deliver well-calibrated and trustworthy decisions, but can also be deployed in the field, are capable of adapting or learning the features of unseen input streams in a secure manner, and operate within the energy budgets of battery-operated devices.”
Rajendran (continued from previous page)
principle, biological brains train internal probabilistic models of the environment such that the degree of surprise between its predictions and observations is minimized, either by adjusting the model parameters or by acting to change the observations themselves.11,12
In parallel, custom hardware based on engineered nanoscale devices and circuits for in-memory computing (IMC) architecture need to be developed. The advantages of IMC architectures for AI acceleration have already been established based on Si CMOS prototypes such as IBM’s TrueNorth13 and Intel’s Loihi14 chips. Going beyond CMOS, a wide variety of nanoscale memristive devices have been studied over the past two decades to efficiently mimic the neuronal and synaptic characteristics required to implement AI models. However, the noisy
About the Author
Bipin Rajendran, Reader in Engineering, King’s College London
Education: BTech (I.I.T. Kharagpur), MS and PhD in Electrical Engineering (Stanford University).
Research Interests: Building algorithms, devices, and systems for brain-inspired computing.
Work Experience: Master Inventor and Research Staff Member at IBM T. J. Watson Research Center in New York (2006–2012) and has held faculty positions in India and the US.

Pubs + Patents: Co-authored >90 papers in peer-reviewed journals and conferences, one monograph, one edited book, and received 59 issued US patents.
Honors & Awards: IBM Faculty Award (2019), IBM Research Division Award (2012), and IBM Technical Accomplishment Award (2010). Elected a senior member of the US National Academy of Inventors in 2019.
Website: https://sites.google.com/site/rajendranbipin/?pli=1 https://orcid.org/0000-0002-2960-6909
References
1. I. Gorton, P. Greenfield, A. Szalay, and R. Williams, Computer, 41 (4), 30 (2008)
2. D. Reinsel, J. Gantz, and J. Rydning, IDC White Paper (2017).
and stochastic imperfections of these devices pose a significant challenge in achieving the same decision-making performance as high-precision software.15,16 The operating characteristics (e.g., conductance, memory switching, stability of programmed states, etc.) of most nanoscale devices exhibit significant device-to-device and within-device stochasticity. Most efforts in the literature devote additional architectural resources to recover the associated accuracy loss due to device imperfections.17–20 A more attractive approach is to use nanoscale device noise and stochasticity as a resource for computation—some recent papers have proposed leveraging nanoscale device stochasticity for combinatorial optimization21 and Bayesian learning.22 This would be particularly relevant for implementing probabilistic AI models based on Bayesian or FEP-based approaches.
To identify the target specifications of the nanoscale computational elements necessary to build efficient AI technologies, consider the design of an on-chip learning engine that supports 100 million neurons and 100 billion synapses integrated into a 2 cm × 2 cm chip, operating 1000 times faster than the human brain but with a power budget of 1 Watt; these targets are 1000 times better than currently available Si neuromorphic chips such as Loihi.14 The metrics in Table I, estimated based on back-of-the-envelope calculations, translate to a device-switching energy of ~2500kBT. There is a huge opportunity for developing novel devices that exploit the properties of novel nanoscale solid state and electrochemical materials that would be key for enabling next-generation AI technologies.
If AI systems based on the above-outlined principles could be built, this would have a transformative impact in a wide swath of embedded applications spanning telecommunications, environmental sustainability, transportation, and security. These platforms would also enable a new class of implantable devices that could monitor the health and well-being of patients in a timely manner, significantly reducing the cost of healthcare delivery.
© The Electrochemical Society. DOI: 10.1149/2.F09231IF
3. A. Tamkin, M. Brundage, J. Clark, and D. Ganguli, CoRR abs/2102.02503, arXiv (2021).
4. J. Jumper, R. Evans, et al., Nature, 596, 583 (2021)
5. P. Dhar, Nat. Mach. Int., 2, 423 (2020)
6. E. Strubell, A. Ganesh, and A. McCallum, ACL, 3645 (2019)
7. K. Quach, The Register.com, Nov, (2020).
8. Editors, Nat. Nano., 15, 507 (2020).
9. R. Schwartz, J. Dodge, N. A. Smith, and O. Etzioni, ACM Comms., 63, 54 (2020).
10. K. Friston, J. Kilner, and L. Harrison, J. Physiol. Paris, 100, 70 (2006).
11. K. Friston, Nat. Rev. Neurosci., 11(2), 127, (2010).
12. C. Buckley, C. S. Kim, S. McGregor, and A. K. Seth, J. Math. Psychol., 81, 55 (2017).
13. P. A. Merolla, J. V. Arthur, et al., Science, 345 (6197), 668 (2014).
14. M. Davies, N. Srinivasa, et al., IEEE Micro, 38, 82 (2018).
15. A. Mehonic, A. Sebastian, et al., Advanced Intell. Sys., 2, (2020).
16. B. Rajendran, A. Sebastian, et al., IEEE Signal Proc. Mag., 36, 97 (2019).
17. D. Joksas, P. Fritas, et al., Nat. Comm., 11, 4273 (2020).
18. S. R. Nandakumar, M. Le Gallo, et al., Front. in Neurosc., May (2020).
19. S. R. Nandakumar, I. Boybat, et al., Sci. Rep., 10, 880 (2020)
20. I. Boybat, M. Le Gallo, et al., Nat. Comm., 9, 2514 (2018).
21. F. Cai, S. Kumar, et al., Nat. Electronics, 3, 409 (2020).
22. T. Dalgaty, N. Castellani, et al., Nat. Electronics, 4, 151 (2021).
48 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org
ATTRIBUTE TARGET Neuron size 60 x 600 nm2 Synapse size 60 x 60 nm2 Device on current 1 – 10 nA Device off current 1 – 10 pA Operating voltage 100 mV Switching time 10 ns Number of states > 32
Table I. Target specifications of nanoscale memristive devices.
Emerging Memory Devices Beyond Conventional Data Storage: Paving the Path for Energy-Efficient Brain-Inspired Computing
by Rashmi Jha
The current state of neuromorphic computing broadly encompasses domain-specific computing architectures designed to accelerate machine learning (ML) and artificial intelligence (AI) algorithms.1 As is well known, AI/ML algorithms are limited by memory bandwidth.2 Novel computing architectures are necessary to overcome this limitation. There are several options that are currently under investigation using both mature and emerging memory technologies. For example, mature memory technologies such as high-bandwidth memories (HBMs) are integrated with logic units on the same die to bring memory closer to the computing units.3 There are also research efforts where inmemory computing architectures have been implemented using DRAMs or flash memory technologies.4,5 However, DRAMs suffer from scaling limitations, while flash memory devices suffer from endurance issues.6,7 Additionally, in spite of this significant progress, the massive energy consumption needed in neuromorphic processors while meeting the required training and inferencing performance for AI/ML algorithms for future applications needs to be addressed.8 On the AI/ML algorithm side, there are several pending issues such as life-long learning, explainability, context-based decision making, multimodal association of data, adaptation to address personalized responses, and resiliency. These unresolved challenges in AI/ML have led researchers to explore brain-inspired computing architectures and paradigms. It is noteworthy that a biological brain naturally addresses these issues while consuming just a fraction of the amount of energy required by a conventional computer.
When it comes to brain-inspired paradigms of computing, memory devices used for storing weights in neuromorphic computers are compared to biological synapses. A biological process engine (PE)
can be considered as an aggregate of neurons connected via synapses. A fundamental difference between neuromorphic PE (shown in Fig.1(a)) and biological PE (shown in Fig.1(b)) is that a biological synapse changes conductance based on learning rules, which reconfigures the signal transmission pathways between neuronal populations.9 This seemingly simplistic approach serves as a basis for biological computing.
But then one ponders why it has been so difficult to replicate the computing paradigms of the brain? Biological synapses are diverse in morphology and functionality. They also demonstrate dynamic behavior on multiple time scales, such as short-term plasticity (STP), which forms the basis of working memory and sensory information filtering.10 Dendritic architectures and distribution of synapses on dendrites also play a critical role in biological computing by modulating signal delays.11 Several reports indicate that data is stored in the form of spatiotemporal clusters of synapses in the brain.12 Additionally, beyond Hebbian learning based on pre- and postneuronal spiking times, a third factor such as neurotransmitters or rewards that convey information about success can play an important role in learning which can be accommodated by biological synapses.13 Conventional memory elements (such as DRAM, SRAM, flash) lack the versatility of biological synapses. This limitation is where the true benefit of emerging memory technologies can be leveraged, as many of the emerging memory devices can be engineered to manifest the “dynamic behavior.”

There are several emerging memory devices that are currently under investigation to replace or complement the conventional memory technologies in neuromorphic architectures.14 This article will discuss resistive random access memory (RRAM) devices as
(continued on next page)

The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 49
Fig. 1. (a) Systolic array-based machine learning (ML) processing unit, (b) Neuro-synaptic processing unit in biological brain showing pyramidal neuron with complex dendritic architecture.
(a) (b)
these are one of the more promising candidates and they have been widely studied in emerging neuromorphic architectures. RRAMs are two-terminal devices in a metal-insulator-metal (MIM) configuration, shown in Fig.2(a). The insulator is usually a metal oxide15. An interfacial layer can be designed to modulate the properties of metal oxide by serving as an oxygen exchange layer. Various dopants in metal oxides have also been widely investigated to achieve the desired switching characteristics.16 These devices can be easily integrated on complementary metal oxide semiconductor (CMOS) platforms in back-end-of-line (BEOL) processing, adding computing value to the passive interconnects. There are two broad categories of RRAMs— filamentary and non-filamentary. In filamentary-RRAMs, the first step involves electroforming by applying positive electroforming voltage with compliance current (Icc) control that leads to the formation of a defect-assisted filament, shown in Fig.2(b). These defects could be oxygen vacancies or metal ions. Then, the first reset is performed by applying negative voltage to retract the filament via a possible redox reaction, shown in Fig. 2(c). Finally, set operation is performed by applying positive voltage to reform the filament with relatively smaller Icc to define the low-resistance state (LRS) (Fig. 2(d)). A subsequent reset operation leads to a high-resistance state (HRS) (Fig. 2(e)). The device can be switched between LRS and HRS with a write endurance of >106 cycles. Multiple resistive states can be achieved by modulating Icc or reset voltages, which enables multi-bit weight storage in a single device, resulting in the densification of memory.17 The resistive states in non-filamentary RRAMs are driven

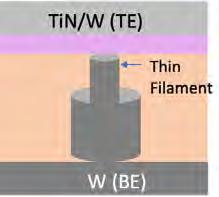
by the modulation of defect states at the oxide/metal interface or in oxide that alters the transport properties of electrons between top and bottom electrodes. Multiple analog resistance states can be achieved in these devices by using different programming conditions.18




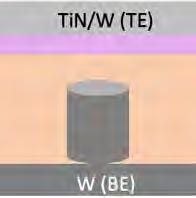
In a neuromorphic hardware, matrix multiplication is one of the most computationally intensive tasks limited by memory bandwidth. RRAM devices have been studied to enable in-memory computing in neuromorphic architectures, which has the potential to accelerate matrix multiplication.19 RRAM devices in a 1 Diode-1 RRAM (1D1R) crossbar configuration are shown in Fig. 3(a). An access diode is necessary to mitigate the sneak current in the crossbars.20 Though 1D1R is highly scalable, the desired specifications for access diodes have been difficult to meet and further research is needed in this area. Therefore, 1 Transistor 1 RRAM (1T1R), where the transistor serves as an access device, is a more practical implementation of RRAM in crossbar arrays currently (Fig. 3(b)). With these RRAM arrays, matrix multiplication is performed in analog fashion where the input voltage is intrinsically multiplied by the conductance state of an RRAM in a cell to result in an output current. The current through each cell is summed on the wire in column, resulting in matrix multiplication. Additional circuitry is needed to sense this current and transform it to the digital domain using analog-to-digital converters, or it is possible to continue processing in the analog domain. These architectures have been used to implement deep neural networks (DNNs).
Just like the brain, a neuromorphic hardware capable of real-time learning and inferencing is highly desirable. However, the training
50 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org
Jha (continued from previous page)
(a) (b) (c) (d) (e)
Fig. 2. RRAM devices: (a) Fabricated state, (b) After electroforming showing thick filament in switching oxide, (c) First reset showing retracted filament, (d) Thin filament growth during set process causing a low-resistance state (LRS), (e) Retracted filament during reset causing a high-resistance state (HRS).
(a) (b)
Fig. 3. RRAM in crossbar array configuration in (a) 1D1R, (b) 1T1R. Input data (v1 to vn) are applied into the array that gets transformed into current (I1 to Im) by multiplication with conductance value of corresponding RRAMs and summation in array
process is usually very complex, requiring additional hardware. Therefore, training and inferencing engines are designed separately to meet the optimum power, performance, area, and cost specifications. Inferencing engines based on RRAM neuromorphic architectures are of much interest for low-power edge-AI applications.21
One of the major drawbacks of RRAMs in neuromorphic architectures for inferencing is the drift in resistance states over time. The LRS and HRS retention over time has been reported to be a function of temperature, Icc, or programming pulse-width.22 The retention of resistive states over time can also be modulated by programming voltages—devices programmed with higher voltages or a higher number of pulses tend to have longer retention compared to devices programmed with lower voltages or a lower number of pulses.
Interestingly, while the time-dependent retention (or dynamic states) of these emerging memory devices is undesirable in a neuromorphic inferencing engine in its current implementation, this characteristic can be considered similar to the STP observed in biological synapses. Additionally, the ability to forget information has been shown to have a positive impact on learning.23 A notable difference between a biological brain and RRAM-based neuromorphic inferencing engines is that a biological brain continues to learn from data even while inferencing. Therefore, time-dependent retention is useful because the system is dynamic. On the other hand, current inferencing engines based on RRAMs are static where states are expected to stay constant over time. A major challenge lies in understanding how we can use the dynamic nature of emerging memory devices to the advantage of neuromorphic systems. Indeed, these STP states of RRAM devices have been leveraged in spiking neural network architectures to demonstrate filtering of noise in sensory data and modified Hebbian learning.24–26 While these preliminary reports are encouraging steps, further work is needed in this area to leverage these unique characteristics. Additionally, currently dynamic states in RRAMs are uncontrolled in nature. Once their applications are established, then they can be engineered to result in the desired performance.
In conclusion, RRAM devices hold promise for applications in neuromorphic computing, though there are some pending challenges that need to be addressed. Beyond their established applications for matrix multiplication in crossbar arrays, it is important to study time-dependent states and to develop techniques for controllably modulating the dynamic states. The reliability of these states needs to be studied as well. Complex dendritic architectures with RRAMs beyond crossbar arrays need to be investigated. A detailed understanding of these dynamic states can help implement cortical circuitries that utilize dynamic synaptic states in diverse distributions using these devices—which can have significant impact on advancing novel paradigms of computing.
Acknowledgment
This work is supported by National Science Foundation under award number ECCS 1926465. ©The Electrochemical Society. DOI: 10.1149/2.F10231IF
About the Author
Rashmi Jha, Professor of Electrical Engineering and Computer Science, University of Cincinnati

Education: BTech (Indian Institute of Technology), MS and PhD (North Carolina State University) in Electrical Engineering.
Research Interests: Artificial intelligence (AI); Low-power neuromorphic systems; CMOS and other emerging logic and memory devices (e.g., RRAM, spintronics, and other memristive devices); On-die sensors; Cross-technology heterogenous integration and modeling; Cybersecurity with emphasis on hardware security; Additive, flexible, and wearable electronics; Nanoelectronics; Neuroscience and neuroelectronics; Bio-inspired computing and systems.
Work Experience: >18 years of experience in solid state electronics and nanoelectronic device design, modeling, fabrication, process integration, electrical characterization, data analysis, circuit design and simulation. Before NCSU, she was Assistant Professor and then Associate Professor in Electrical Engineering and Computer Science at the University of Toledo. Before that, she worked as a Process Integration Engineer at IBM’s Semiconductor Research and Development Center.
Pubs + Patents: >106 peer-reviewed publications; 13 US patents. Honors & Awards: AFOSR Summer Faculty Fellowship Award (2017); NSF CAREER Award (2013)
Website: https://researchdirectory.uc.edu/p/jhari https://orcid.org/0000-0002-2656-5945
References
1. V. Sze, Y. -H. Chen, T. -J. Yang, and J. S. Emer, Proceedings of the IEEE, 105, 2295 (2017).
2. M. Davies, et al., IEEE Micro, 38, 82 (2018)
3. Y.-C. Kwon, et al , 2021 IEEE International Solid- State Circuits Conference (ISSCC), San Francisco, CA, USA, 350 (2021)
4. F. Gao, G. Tziantzioulis, and D. Wentzlaff, MICRO ‘52: Proceedings of the 52nd Annual IEEE/ACM International Symposium on Microarchitecture, 100 (2019)
5. H.-W. Hu, et al., 2022 IEEE International Solid- State Circuits Conference (ISSCC), San Francisco, CA, USA, 138 (2022)
6. S. Shiratake, 2020 IEEE International Memory Workshop (IMW), Dresden, Germany, 1 (2020)
7. C. Zambelli, R. Micheloni, and P. Olivo, 2019 IEEE 11th International Memory Workshop (IMW), Monterey, CA, USA, 1 (2019).
8. J. Zhang, K. Rangineni, Z. Ghodsi, and S. Garg, DAC ‘18: Proceedings of the 55th Annual Design Automation Conference, 19 (2018)
9. N. Spruston, Nat. Rev. Neurosci., 9, 206 (2008)
10. L. Abbott and S. Nelson, Nat. Neurosci., 3 (Suppl 11), 1178 (2000)
11. P. Poirazi and B. W. Mel BW, Neuron., 29 (3), 779 (2001)
12. A. Govindarajan, R. Kelleher, and S. A. Tonegawa, Nat. Rev. Neurosci. 7, 575 (2006).
13. See, for example: Ł. Kuśmierz, T. Isomura, and T. Toyoizumi, Curr. Opin. Neurobiol., 46, 170 (2017)
14. A. Sebastian, et al Nat., Nanotechnol., 15, 529 (2020)
15. B. Govoreanu, et al., 2011 International Electron Devices Meeting, Washington, DC, USA, 31.6.1 (2011).
16. B. Long, et al., ECS Trans., 53, 115 (2013)
17. O. Golonzka, et al., 2019 Symposium on VLSI Technology, Kyoto, Japan, T230 (2019).
18. B. Long, Y. Li, and R. Jha, IEEE Electron. Device Lett., 33 (5), 706 (2012).
19. W. Wan, et al., Nature, 608, 504 (2022)
20. H. Tsai, et al., J. Phys. D: App. Phys., 51 (28), 283001 (2018)
21. Z. Li, et al., IEEE J. Solid-State Circuits, 56 (4), 1105 (2021).
22. Y. Y. Chen, et al., 2013 IEEE International Electron Devices Meeting, Washington, DC, USA, 10.1.1 (2013)
23. T. J. Ryan and P. W. Frankland, P.W., Nat. Rev. Neurosci., 23, 173 (2022).
24. T. J. Bailey, A. J. Ford, S. Barve, J. Wells, and R. Jha, IEEE Trans. Very Large Scale Integ. (VLSI) Syst., 28 (11), 2410 (2020)
25. T. Chang, S.-H. Jo, and W. Lu, ACS Nano, 5 (9), 7669 (2011)
26. Z. Shen, et al., Nanomaterials (Basel), 10 (8), 1437 (2020).
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 51
ECS Japan Section
On December 16, 2022, the ECS Japan Section co-sponsored an anniversary event for the 75th Anniversary of the Transistor organized by the IEEE Electron Devices Society Japan Joint Chapter in Tokyo. Professor Hiroshi Iwai, Councilor of the ECS Japan Section, presented a special talk titled “75th Anniversary of the Transistor: Origin of the Micro/Nanoelectronics toward SuperIntelligent Society.” Some 59 participants joined the onsite meeting at the University of Tokyo.

The ECS Japan Section Executive Committee meeting took place online on December 3, 2022. The new slate of officers began their 2023–2024 terms in January 2023. The new officers are:
Section Chair – Yasushi Idemoto, Tokyo University of Science
1st Vice Chair – Seiichiro Higashi, Hiroshima University
2nd Vice Chair – Yasushi Katayama, Keio University
Section Secretary/Treasurer – Wataru Sugimoto, Shinshu University
Newly elected ECS Japan Section Executive Committee members meet outgoing officers online on December 3, 2022; (top row from left to right)

2022 Section Chair Seiichi Miyazaki, Member-at-Large Osamu Nakatsuka, and 2023 Section Chair Yasushi Idemoto; (second row from left to right)

Member-at-Large Masao Sakuraba, First Vice Chair Seiichiro Higashi, and Section Secretary/Treasurer Wataru Sugimoto; (third row from left to right) Member at Large Minoru Inaba, 2nd Vice Chair Yasushi Katayama, and Councilor Masayoshi Watanabe
BENEFITS:
• Access to innovative research
•
52 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org
NEWS
NEWS
SECTION
SECTION
Prof. Hiroshi Iwai, Councilor of the ECS Japan Section, gives a special talk at the “75th Anniversary of the Transistor” event at the University of Tokyo on December 16, 2022.
• Global reach
Open New Doors— Join ECS Sections
more information, contact customerservice@electrochem.org
Networking and recognition
For
ECS Japan Section 2022 and 2023 Section Chairs and Secretaries meet on December 8, 2022, at Tokyo University of Science; (from left to right) 2023 Secretary/Treasurer Wataru Sugimoto, 2023 Section Chair Yasushi Idemoto, 2022 Section Chair Seiichi Miyazaki, and 2022 Secretary/Treasurer Osamu Nakatsuka
Section Name Section Chair
Arizona Section Candace Kay Chan, Chair
Brazil Section Raphael Nagao de Souza, Chair
Canada Section Heather Andreas, Chair
Chile Section José H. Zagal, Chair
China Section Yongyao Xia, Chair
Detroit Section Eric Anderson, Chair
Europe Section Open

Georgia Section Open
India Section Sinthai A. Ilangovan, Chair
Israel Section Daniel Mandler, Chair
Japan Section Seiichi Mayazaki, Chair
Korea Section Won-Sub Yoon, Chair

Mexico Section Carlos E. Frontana-Vázquez, Chair
Mid-America Section Nosang Vincent Myung, Chair
National Capital Section Open
New England Section Sanjeev Mukerjee, Chair
Pacific Northwest Section Corie Cobb, Chair
Pittsburgh Section Open
San Francisco Section Gao Liu, Chair
Singapore Section Zichuan J Xu, Chair
Taiwan Section Chi-Chang Hu, Chair
Texas Section
Twin Cities Section
Jeremy P. Meyers, Chair
Victoria “Vicki” Gelling, Chair
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 53 SECTION NEWS
NEWS
Visit www.electrochem.org/sections to learn more about ECS sections. Research is meant to be shared. Make a donation today!
SECTION
Section Leadership
LEARN MORE ?
AWARDS PROGRAM AWARDS PROGRAM
Awards, Fellowships, Grants

The Honors & Awards Program recognizes outstanding technical achievements in electrochemistry and solid state science and technology, and exceptional service to the Society, through ECS Society, Division, Section, and Student Awards. Highlights follow.

Society Awards
The ECS Toyota Young Investigator Fellowship, established in 2015 in partnership with the Toyota Research Institute of North America, encourages young professionals and scholars to pursue research into batteries, fuel cells and hydrogen, and future sustainable technologies. Each year, at least one candidate receives the fellowship restricted grant of no less than $50,000* to conduct the proposed research within one year, and a one-year complimentary ECS membership. Recipients must present at a Society biannual meeting and publish their research in a relevant ECS journal within 24 months of receiving the award. Materials are due January 31 annually.
The Charles W. Tobias Award, established in 2003, recognizes outstanding scientific and/or engineering work in fundamental or applied electrochemistry or solid state science and technology by a young scientist or engineer. The award consists of a framed certificate; $5,000 prize; ECS Life Membership; complimentary meeting registration; and assistance with travel to the designated meeting. Materials are due by October 1, 2023.
The Edward Goodrich Acheson Award was established in 1928 for distinguished contributions to the advancement of any of the objects, purposes, or activities of The Electrochemical Society. The award consists of a gold medal; a plaque with bronze replica of the medal; $10,000 prize; Society Life Membership; and complimentary meeting registration. Materials are due by October 1, 2023.


The Henry B. Linford Award for Distinguished Teaching was established in 1981 for excellence in teaching in subject areas of interest to the Society. The award consists of a silver medal and a plaque containing a bronze replica thereof; a $2,500 prize; Society Life Membership; and complimentary meeting registration. Materials are due by April 15, 2023.

Leadership Circle Awards, established in 2002 to honor and to thank our electrochemistry and solid state science partners, are granted in the anniversary year that an institutional member reaches a milestone level. Awardees receive a commemorative plaque and recognition on the ECS website and in Interface. Nominations are not accepted.

The Vittorio de Nora Award, established in 1971, recognizes distinguished contributions to the field of electrochemical engineering and technology. The award consists of a gold medal and a plaque that contains a bronze replica thereof; $7,500; Society Life Membership; and complimentary meeting registration. Materials are due by April 15, 2023.
Division Awards
Biannual Meeting Travel Grants are awarded for each Society biannual meeting. Many ECS divisions and sections offer travel grants to undergraduates, graduate students, postdoctoral researchers, and young professionals and faculty presenting papers at ECS biannual meetings. The awards consist of financial support ranging from complimentary meeting registration to luncheon/ reception tickets, travel support, and more. Divisions and sections maintain their own application requirements. 244th ECS Meeting Travel Grant applications are accepted from April 7 through June 26, 2023.
The Electrodeposition Division Early Career Investigator Award, established in 2015, recognizes an outstanding early career researcher in the field of electrochemical deposition science and technology. The award consists of a scroll; $1,000 prize; and complimentary meeting registration. Materials are due by April 1, 2023.
The Electronics and Photonics Division Award was established in 1969 to encourage excellence in electronics research and outstanding technical contributions to the field of electronics science. The award consists of a framed certificate; $1,500 prize; and ECS Life Membership or up to $1,000 to facilitate travel to the designated meeting. Materials are due by August 1, 2023.

54 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org
Visit www.electrochem.org/awards for more information.
AWARDS AWARDSAWARDSPROGRAM AWARDS PROGRAM
The Energy Technology Division Research Award was established in 1992 to encourage excellence in energyrelated research. The award consists of a framed certificate; $2,000 prize; and membership in the ECS Energy Technology Division for as long as the recipient is an ECS member. Materials are due by September 1, 2023.
The Energy Technology Division Supramaniam Srinivasan Young Investigator Award, established in 2011, recognizes and rewards an outstanding young researcher in the energy technology field. The award consists of a framed certificate; $1,000 prize; and complimentary meeting registration. Materials are due by September 1, 2023.
The Energy Technology Division Walter van Schalkwijk Award in Sustainable Energy Technology was established in 2021 to recognize and reward researcher scientists, academicians, and entrepreneurs who make innovative and transformative contributions to sustainable energy technologies (devices, materials, and/or processes). The award consists of a framed certificate and monetary prize equal to 1/25th of the endowment with a maximum of $2,500. Materials are due by April 15, 2023.
The Nanocarbons Division Richard E. Smalley Research Award was established in 2006 to encourage excellence in fullerene, nanotube, and carbon nanostructure research. The award is intended to recognize, in a broad sense, those persons who have made outstanding contributions to the understanding and applications of fullerenes. Awardees receive a framed certificate, $1,000 prize, and up to $1,500 in travel assistance. Materials are due by September 1, 2023.
The Nanocarbons Division SES Research Young Investigator Award, established in 2007, recognizes and rewards an outstanding young researcher in the field of fullerenes, carbon nanotubes, and carbon nanostructures. The award consists of a framed certificate; $500 prize; and complimentary meeting registration. Materials are due by September 1, 2023.
The Physical and Analytical Electrochemistry Division David C. Grahame Award was created in 1981 to encourage excellence in physical electrochemistry research and to stimulate publication of high-quality research papers in the Journal of The Electrochemical Society. The award consists of a framed certificate and $1,500 prize. Materials are due by October 1, 2023.
Section Awards
The Pacific Northwest Section Electrochemistry Research Award Sponsored by Gamry Instruments was established in 2021 to recognize excellence in electrochemistry and solid state science and technology research. The award consists of a certificate and $1,000 prize. Materials are due by July 15, 2023.
Student Awards
ECS Summer Fellowships, established in 1928, assist students pursuing research from June through August in a field of interest to ECS. Up to four summer fellowships are awarded each year: the Edward G. Weston Fellowship, Joseph W. Richards Fellowship, F. M. Becket Fellowship, and H. H. Uhlig Fellowship. Recipients receive $5,000 to support their research and publication of a summary report in the award year’s Interface winter issue. Materials are due by January 15 of each year.
The Colin Garfield Fink Fellowship, first awarded in 1962, assists a postdoctoral scientist/researcher pursue research from June through August in a field of interest to the Society. The award consists of $5,000 and publication of a summary report in the award year’s Interface winter issue. Materials are due by January 15 of each year.





The ECS General Student Poster Session Awards, established in 1993, acknowledge the quality and thoroughness of candidates’ work; the originality and independence of their contributions; the significance and timeliness of research results; and the depth of the understanding of the research topics and their relationship to the Society’s fields of interest. Three awards are given at each Society biannual meeting. First place receives $1,500; 2nd place receives $1,000; and 3rd place receives $500. Awardees are also recognized with a certificate and announcement in the Interface issues accompanying the respective meeting’s “Biannual Meeting Highlights” article. Students must submit abstracts to the General Student Poster Session by the biannual meeting abstract deadline to be considered for the awards. The 244th ECS Meeting abstract submission deadline is April 7, 2023.
The ECS Outstanding Student Chapter Award (formerly The Gwendolyn B. Wood Section Excellence Award) was launched in 2012 to recognize distinguished student chapters that demonstrate active participation in the Society’s technical activities; establish community and outreach activities in the areas of electrochemical and solid state science and engineering education; and create and maintain a robust membership base. The winning Outstanding Student Chapter receives a recognition plaque and certificates; $1,000; and award recognition and chapter group photo in Interface or other electronic communications. Up to two Chapters of Excellence are awarded. Materials are due by April 15, 2023.
The Energy Technology Division Graduate Student Award
Sponsored by BioLogic, established in 2012, recognizes and rewards promising young engineers and scientists in fields pertaining to this division. The award consists of a framed certificate; $1,000; complimentary student meeting registration; and complimentary admission to the division’s business meeting. Materials are due by September 1, 2023.


The Georgia Section Student Award, established in 2011, recognizes academic accomplishment in any area of science or engineering in which electrochemical and/or solid state science and technology is the central consideration. Recipients—PhD students at universities within the Georgia Section—are nominated by university faculty members. The award consists of a $500 prize. Materials due by August 15, 2023.
(continued on next page)
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 55
AWARDS PROGRAM AWARDS PROGRAM
(continued from previous page)
The Industrial Electrochemistry and Electrochemical Engineering Division H. H. Dow Memorial Student Achievement Award, established in 1990, recognizes promising young engineers and scientists in the field of electrochemical engineering and applied electrochemistry. The award consists of a framed certificate and $1,000 prize for expenses associated with the recipient’s education or research project (i.e., tuition, books, equipment, or supplies). Materials due by September 1, 2023.
The Industrial Electrochemistry and Electrochemical Engineering Division Student Achievement Award, established in 1989, recognizes promising young engineers and scientists in the field of electrochemical engineering. The award consists of a framed certificate and $1,000 prize. Materials due by September 1, 2023.


*US dollars
Award Winners
Join us in celebrating your peers as we congratulate them all! The following awards are part of the ECS Honors & Awards Program, which has recognized professional and volunteer achievement within our multi-disciplinary sciences for decades.
Society Awards
Allen J. Bard Award
Joseph Hupp is the Charles E. and Emma H.Morrison Professor of Chemistry at Northwestern University. His research centers on energy- and defense-relevant materials chemistry, including design and synthesis of materials for chemical separations, chemical catalysis, electrocatalysis, light-to-electrical energy conversion, artificial photosynthesis, storage and release of molecular hydrogen, and capture and destruction of chemical warfare agents.
Professor Hupp, a native of rural western New York State, was introduced to electrochemical research as an undergraduate at Houghton College when evaluating candidate electrode materials for heart pacers. He completed his PhD in Chemistry at Michigan State University in 1983 under the late Mike Weaver. After a postdoc with Thomas J. Meyer at the University of North Carolina, he joined the faculty of Northwestern in 1986.
A longtime ECS member, Prof. Hupp is a Fellow of the American Academy of Arts and Sciences, American Chemical Society, Materials Research Society, American Association for the Advancement of Science, and Royal Society of Chemistry. He has mentored some 200 PhD students, postdoctoral fellows, and visiting scholars, and about 40 undergraduate research students. Alumni of his group are on the faculties of research universities and liberal arts colleges across the US and worldwide. Recognized by Clarivate Analytics as one of the world’s most highly cited chemists, Prof. Hupp’s research findings are described in nearly 700 peer-reviewed articles and 29 patents.
Gordon E. Moore Medal for Outstanding Achievement in Solid State Science & Technology
Fred Roozeboom is Emeritus Professor in the Inorganic Membranes Group at Universiteit Twente (UT) and a consultant for high-tech industries (tier-1 and SMEs [smallandmedium-sizedenterprises]).Since 2004, his research has focused on selective atomic layer etching (ALE) and atomic layer deposition (ALD), Li-ion batteries, extreme ultraviolet (EUV) optics lifetime, and CO2 capture.
Prof. Roozeboom received his MSc cum laude at Universiteit Utrecht in 1976 and PhD on topics in catalysis from UT in 1980. He researched zeolite catalysis with ExxonMobil in Baton Rouge and Rotterdam from 1980 to 1983. At Philips Research (now NXP Research) from 1983 to 1997, he worked on III–V semiconductors, integrated circuit (IC) metallization, and magnetic thin films. From 1997 to 2009, he led a team there studying 3D-silicon-based passive integration and via hole (TSV) technology for wireless communication and power management. Prof. Roozeboom received the NXP Bronze Invention of the Year 2007 Award and became a Research Fellow. From 2007 to 2021, as a part-time professor at the Technische Universiteit Eindhoven, he specialized in thin-film technology (plasma etching and atomic layer processing). Concurrently, starting in 2009, Prof. Roozeboom researched the industrialization of spatial ALD and related processing at the TNO Holst Centre. TNO’s spatial processing team received the 2011 European Association of Research & Technology Organizations (EARTO) Innovation Award. He joined UT in 2021.

Prof. Roozeboom is the author or coauthor of over 200 publications on chemistry and physics (h-index of 42), five book chapters, 39 granted US patents, editor or coeditor of 51 conference proceedings on semiconductor processing, and Executive Editor of the open access Atomic Layer Deposition International Journal. In 2014, he was named Fellow of The Electrochemical Society. He was or is active in several conference committees (AVS, DPS-Japan, ECS, IEEE, and the Materials Research Society [MRS]), and is a member of SEMI Europe’s Semiconductor Technology Programs Committee. He is an ECS Electronics and Photonics Division Member-at-Large, was a member of the European Nanoelectronics Initiative (ENIAC) Advisory Committee to the European Commission, and Meeting Chair of the 2003 MRS Fall Meeting.
John B. Goodenough Award of the Electrochemical Society

Arumugam Manthiram is Cockrell Family Regents Chair in Engineering and Professor, Walker Department of Mechanical Engineering, at the University of Texas at Austin (UT Austin). His long-term friendship and collaboration with Prof. Goodenough began as a postgraduate researcher with Prof. Goodenough at Oxford University in 1985, and extended to delivering the 2019 Chemistry Nobel Prize Lecture in Stockholm on the professor’s behalf.
Prof. Manthiram received his PhD in Solid State Chemistry from the Indian Institute of Technology–Madras (IIT–Madras) in 1980.

56 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org
AWARDS AWARDSAWARDSPROGRAM AWARDS PROGRAM
Named Assistant Professor in the Walker Department of Mechanical Engineering at UT Austin in 1991, he was promoted to the rank of Professor in 2000. He served as Director of the Texas Materials Institute and the Materials Science and Engineering Graduate Program from 2011 through 2022.

More than 300 students and postdoctoral researchers, including 69 PhD students have received research training from Prof. Manthiram. Of them, 55 are now faculty around the world; several hold leadership positions in industry. His current research group includes about 35 graduate students and postdoctoral fellows. The author of more than 900 journal articles with over 98,000 citations (h-index of 155), Prof. Manthiram has 20 issued patents. He is one of 6,200 scientists and engineers in all fields in the world included as Clarivate Highly Cited Researchers every year since 2017.
Numerous honors mark Prof. Manthiram’s distinguished contributions to science. He is a Fellow of The Electrochemical Society, Royal Society of Chemistry, Materials Research Society, American Ceramic Society, American Association for the Advancement of Science, and World Academy of Materials and Manufacturing Engineering, and elected academician of the World Academy of Ceramics. He has received the 2021 ECS Battery Division Technology Award; 2020 ECS Henry B. Linford Award for Distinguished Teaching; 2020 International Battery Association Research Award; 2018 Da Vinci Award; 2016 Billy & Claude R. Hocott Distinguished Centennial Engineering Research Award; 2015 IIT–Madras Distinguished Alumnus Award; 2014 ECS Battery Division Research Award; 2012 UT Austin Outstanding Graduate Teaching Award, among many other awards.
Since joining ECS in 1995, Prof. Manthiram has volunteered in positions that include Chair of the Texas Section (2006–2007) and the Battery Division (2010–2012), and on many ECS committees, including the Editorial Advisory Board, Symposium Planning Advisory Board, Interdisciplinary Science and Technology Subcommittee, and various award subcommittees. He founded the ECS University of Texas at Austin Student Chapter in 2006 and continues as its Faculty Advisor today.
Norman Hackerman Young Author Award (2021)
For the paper, Entropy Measurements of Li-Ion Battery Cells with Li- and Mn-Rich Layered Transition Metal Oxides via Linear Temperature Variation [J. Electrochem. Soc., 168, 120502 (2021)]
Franziska Friedrich is a battery specialist for BMW, where her research primarily focuses on all solid state batteries. She received her BS in Chemistry and Biochemistry in 2016 and her MSc in 2018 from Ludwig-Maximilians-Universität München. Her undergraduate studies focused on electrochemistry, inorganic solid state chemistry, and materials sciences. She received her PhD summa cum laude in 2022 from the Technische Universität München (TUM). There, in Prof. Hubert A. Gasteiger’s Technical Electrochemistry Group, her research centered on cathode active materials used for lithium-ion batteries. Dr. Friedrich investigated the aging phenomena of Ni-rich cathodes and published a Journal of the European Ceramic Society Editor’s Choice article in this research field. She also studied hysteresis phenomena in Li-and Mn-rich NCMs with potentiometric entropy measurements and isothermal micro-calorimetry to understand the effect of the hysteresis on the heat evolution of this type of cathode active material.

Division Awards
Dielectric Science and Technology Thomas D. Callinan Award
Chennupati Jagadish is a Distinguished Professor and Head of the Semiconductor Optoelectronics and Nanotechnology Group at the Research School of Physics, Australian National University. He currently serves as President of the Australian Academy of Science and in the past served as President of the IEEE Photonics Society, IEEE Nanotechnology Council, and Australian Materials Research Society. Prof. Jagadish is the Editor-in-Chief of Applied Physics Reviews, editor of two book series, and serves on editorial boards of 20 other journals. He has published more than 1,020 research papers (730 journal papers); holds seven US patents; coauthored a book; coedited 15 books; and edited 13 conference proceedings and 20 special journal issues. Prof. Jagadish is a Fellow of 12 science and engineering academies in Australia, the US, the UK, Europe, and India, and 14 professional societies, including ECS, IEEE, MRS, and APS. He has received many awards, including the IEEE Pioneer Award in Nanotechnology and Photonics Society Engineering Achievement Award; Optica (formerly OSA) Nick Holonyak, Jr. Award; International Union of Global Materials Research Societies (IUMRS) Sômiya Award; Welker Award; and Walter Boas, W. H. (Beattie) Steel, UNESCO, and Thomas Ranken Lyle Medals. Prof. Jagadish has received Australia’s highest civilian honor, the Companion of the Order of Australia, for his contributions to physics and engineering, in particular nanotechnology.
Electronics and Photonics Division Award
Jean-Michel Hartmann is a CEA Fellow at CEA-Leti (Commissariat à l’énergie atomique et aux énergies alternatives), operational manager of Leti’s team working on group-IV epitaxy, and his department’s scientific director. His research focuses on the reduced pressure chemical vapor deposition of group-IV semiconductors for nanoelectronics and optoelectronics.
Dr. Hartmann completed his PhD in Physics at Université Grenoble Alpes in 1997. His research focused on the solid source molecular beam epitaxy of CdTe/MnTe and CdTe/MgTe heterostructures for optical purposes. As a Post-doctoral Fellow at Imperial College from 1997 to 1999, he investigated the gas source molecular beam epitaxy of Si/SiGe heterostructures for modulated-doping field effect transistors (MODFETs). Dr. Hartmann began at CEA-Leti in 1999 as a Research Engineer. In 2007, he was named CEA Senior Expert, then CEA Research Director and CEA Fellow in 2016.

Dr. Hartmann won the 2017 Helmholtz International Fellow Award in recognition of over 10 years of collaboration with the Forschungszentrum Jülich (FZJ), initially on European projects and subsequently under the CEA-FZJ framework agreement. The collaboration has resulted in approximately 70 joint papers in the fields of nanoelectronics and photonics, with the latest focusing on GeSn-based components.
(continued on next page)
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 57
Photo: Australian Academy of Science
AWARDS PROGRAM AWARDS PROGRAM
(continued from previous page)
Energy Technology Division Graduate Student Award sponsored by Bio-Logic
Yirui (Arlene) Zhang is a Postdoctoral Scholar in the Dionne Lab at Stanford University. She completed her PhD in 2022 with Prof. Yang Shao-Horn in the Department of Mechanical Engineering at the Massachusetts Institute of Technology. She earned her BS from Tsinghua University, also in Mechanical Engineering. Her thesis focused on electrochemical energy storage and conversion, including Li-ion batteries and electrocatalysis. She developed in situ characterizations to probe the electrode-electrolyte interface and to understand the interfacial molecular structures and electrochemical reactions. Dr. Zhang leveraged the physical chemistry of liquid electrolytes and tuned the molecular structure at the outer Helmholtz layer to notably improve the stability and kinetics of electrochemical reactions. Her work has been published in Energy & Environmental Science, Nature Catalysis, and other publications.

Energy Technology Division Research Award
Adam Weber is a Senior Scientist and Leader of the Energy Conversion Group at Lawrence Berkeley National Laboratory and co-Director of the Million Mile Fuel Cell Truck consortium. His current research involves understanding and optimizing fuel cell and electrolyzer performance and lifetime, including component and ionomer structure/function studies using advanced modeling and diagnostics, understanding flow batteries for grid-scale energy storage, and analysis of solar-fuel generators and CO2 reduction.
Dr. Weber holds BS and MS degrees from Tufts University and a PhD in Chemical Engineering under the guidance of John Newman at the University of California, Berkeley. He is the coauthor of over 200 peer-reviewed articles and 11 book chapters on fuel cells, flow batteries, and related electrochemical devices. Dr. Weber has developed many widely used models for fuel cells and their components, and he has been invited to present his work at international and national meetings. He is the recipient of awards that include a Fulbright scholarship to Australia; 2012 Presidential Early Career Award for Scientists and Engineers (PECASE); 2014 ECS Charles W. Tobias Young Investigator Award; 2016 Sir William Grove Award from the International Association for Hydrogen Energy; and a 2020 R&D 100 Award for microelectrode development. Dr. Weber is a Fellow of The Electrochemical Society and the International Association of Advanced Materials.

Energy Technology Division Supramaniam Srinivasan Young Investigator Award
Kelsey Stoerzinger is an Assistant Professor in the School of Chemical, Biological, and Environmental Engineering at Oregon State University. Her research group focuses on designing and understanding electrocatalysts that are selective and efficient in converting and storing renewable energy and leveraging its use for molecular transformations and resource recovery. Stoerzinger holds a joint appointment at Pacific Northwest National Laboratory, where she was a Linus Pauling Distinguished Postdoctoral Fellow until 2018. She completed her Materials Science and Engineering PhD in 2016 at the Massachusetts Institute of Technology, supported by a National Science Foundation Graduate Research Fellowship.
Prof. Stoerzinger received an MPhil in Physics from the University of Cambridge as a Churchill Scholar and a BS from Northwestern University. She has received the MRS Nelson “Buck” Robinson Science and Technology Award for Renewable Energy; ISE Prize for Electrochemical Materials Science; and the Intel Rising Star Faculty, National Science Foundation CAREER, and DOE (Department of Energy) Early Career Research Awards, in addition to recognition for her contributions as a teacher and advisor.

High-Temperature Energy, Materials, & Processes Division Subhash Singhal Award
Tatsuya Kawada is Professor and Dean of the Graduate School of Environmental Studies at Tohoku University, Japan. He received his MSc from the University of Tokyo Graduate School of Engineering in 1986, and joined the National Chemical Laboratory for Industry at the National Institute of Advanced Industrial Science and Technology (AIST), Ministry of International Trade and Industry (MITI). He started research on ion-conducting materials and soon became involved in research on solid oxide fuel cell materials.
Prof. Kawada completed his PhD in Engineering from the University of Tokyo in 1995, and became Associate Professor at the Research Institute of Scientific Measurements, Tohoku University. There, he worked on the materials for gas sensors, high temperature photovoltaic cells, ion emission sources, and solid oxide fuel cells. In 2006, Tohoku University appointed him Professor at the Graduate School of Environmental Studies. Since 2009, Prof. Kawada has served as Principal Researcher of the Tohoku University Group of the New Energy and Industrial Technology Development Organization (NEDO) SOFC National Project. He was head of the Tohoku branch of The Electrochemical Society of Japan in 2017–2018 and board member in 2020–2021. Prof. Kawada was named President of the SOFC Society of Japan in 2022.

58 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org
Photo: Hannah O'Leary
AWARDS AWARDSAWARDSPROGRAM AWARDS PROGRAM
Industrial Electrochemistry and Electrochemical Engineering Division
H. H. Dow Memorial Student Achievement Award
Bairav Sabarish Vishnugopi is a PhD candidate in the School of Mechanical Engineering at Purdue University. His research in Purdue’s Energy and Transport Sciences Lab (ETSL) focuses on understanding the physicochemical phenomena and coupled mechanistic processes that influence the electrochemical performance, degradation, and safety of Liion and beyond Li-ion chemistries. Part of his PhD research examined the electrochemical, transport, mechanics, and thermal interactions in various battery systems, including Li-ion, Li-sulfur, and solid state Li-metal batteries. A major focus of his research is investigating the origin and propagation of disparate failure modes in Li-metal batteries. His work on Li-metal batteries with liquid electrolytes interrogates the chemo-mechanical and transport mechanisms underlying the growth of dendrites and solid electrolyte interphase failure. In the context of solid state batteries, he has analyzed a wide range of degradation modes, including filament evolution, contact loss, and interphase growth, and developed a systematic connection to fundamental descriptors involving the morphological, kinetic, and thermal stability of solid-solid interfaces. Specifically, his research aims to decipher the role of such degradation pathways under operational extremes like fast charging. He has published over 20 journal papers on different energy storage topics. He received the R. H. Kohr Graduate Student Fellowship in Mechanical Engineering for his research contributions through physics-based modeling, simulation, and analysis.
Industrial Electrochemistry and Electrochemical Engineering Division Student Achievement Award

Lauren Clarke is a PhD candidate in the Department of Chemical Engineering at the Massachusetts Institute of Technology (MIT). As a member of Prof. Fikile Brushett’s research group, she uses a combination of modeling and experiments to understand the impact of material properties and operating parameters on the performance of electrochemical CO2 separation systems. More generally, her work articulates key technical metrics for efficient, durable, and economically feasible CO2 separations.
Clarke received her MS in Chemical Engineering Practice from MIT in 2020, where she completed a one-semester industrial internship at the Shell Technology Center and Emirates Global Aluminium. She received her BS and MSc in Chemical Engineering from the University of North Dakota (UND) in 2016 and 2018, respectively. Her MSc research under Prof. Gautham Krishnamoorthy focused on implementing high-performance preconditioners and solvers into a multiphase flow simulation code to reduce computation time and improve performance. At UND, Clarke was a student athlete and four-year member of the women’s volleyball team.

Nanocarbons Division Robert C. Haddon Research Award
Francis D’Souza is currently Regents Professor of Chemistry and Materials Science and Engineering at the University of North Texas (UNT) and is part of UNT’s Applied Materials and Manufacturing Processing Institute. Prior to joining UNT in 2011, he was Professor of Chemistry at Wichita State University (WSU). He received his PhD from the Indian Institute of Science, and held postdoctoral positions at the University of Houston and the Université de Bourgogne.
Dr. D’Souza’s research covers a wide range of chemistry, nanophotonics, electrochemistry, and materials science. Principal research interests include supra and nanomolecular chemistry of photosensitizer-carbon nanomaterials, advanced functional materials for light energy harvesting and photovoltaics, electrochemical and photochemical sensors and catalysts, and nanocomposite hybrid materials for energy storage and utilization. Dr. D’Souza has authored or coauthored over 475 publications, given over 400 conference talks, and edited 10 Handbooks on Carbon Nanomaterials, resulting in over 21,000 citations with a cumulative h-index of 74.
Dr. D’Souza has received honors and awards that include Fellow of The Electrochemistry Society and Royal Society of Chemistry; Fulbright Specialist Scholar; ACS-DFW Section Doherty Research Award; Chemical Research Society of India Medal; GIAN Fellow of the Government of India; Japan Society for the Promotion of Science Fellow; and the WSU Excellence in Research Award. Recognition from UNT includes the Research Leadership Award, Toulouse Scholar Award, Regents Professor, and Distinguished Professor. An active ECS member since 1993, Dr. D’Souza was an ECS Board of Directors member from 2004 to 2008. He has previously served as Chair, Vice Chair, Secretary, and Treasurer of the ECS Nanocarbon Division and is currently a Member-at-Large of that division. He was instrumental in establishing and securing endowment monies for the Nanocarbon Division Richard Smalley Research and SES Young Investigator Awards. He has served as Chair or member of several Society-level committees, including the Honors and Awards Committee; the Fellow Subcommittee, and the Acheson, Bard, Callinan, Haddon, Smalley, and Wagner Award Subcommittees. He currently serves as member of the Meetings and Vittorio de Nora Award subcommittees. He has co-organized over 40 symposia for the Society’s fall and spring biannual meetings. Dr. D’Souza has also served for the last 10 years as Technical and Associate Editor of the ECS Journal of Solid State Science and Technology

Physical and Analytical Electrochemistry Division David C. Grahame Award

Keith Stevenson led the development of a new graduate-level research and innovation institute in Moscow, Russia, having served as Provost, Full Professor, and Founder of the Center for Energy Science and Technology (CEST) from 2014 to 2022. He has also designed a modern material science and engineering education program for MS and PhD students. This program delivers an
(continued on next page)
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 59
AWARDS PROGRAM AWARDS PROGRAM
(continued from previous page)
interdisciplinary mix of engineering and natural sciences; and involves an industrial immersion and entrepreneurship and innovation components. Dr. Stevenson’s research interests aim to elucidate and control chemistry at solid/liquid interfaces vital to many emerging energy storage and energy conversion technologies.
Dr. Stevenson received his PhD in 1997 from the University of Utah under the supervision of Professor Henry S. White Subsequently, he held a postdoctoral appointment at Northwestern University (1997–2000) with Joseph T. Hupp; and a professorial appointment from 2000 to 2015 at the University of Texas at Austin. To date, he has published over 350 peer-reviewed publications, 14 patents, and six book chapters. He has received the Society of Electroanalytical Chemistry’s 2021 Charles N. Reilley Award and 2006 Young Investigator Award; 2012 Kavli Fellow Award; 2004 Conference of Southern Graduate Schools New Scholar Award; and 2002 NSF CAREER Award.

ECS AWARDS & RECOGNITION CEREMONY
SECTION AWARDS
Pacific Northwest Section Electrochemistry Research Award (2022)

Eric Dufek is Department Manager for the Idaho National Laboratory (INL) Energy Storage & Electric Transportation Department, overseeing more than 40 research scientists, engineers, postdoctoral researchers, and interns. The department focuses on advanced transportation systems with an emphasis on the use, analysis, and controls for electric vehicle infrastructure; the development, evaluation, and identification of technology gaps for advanced battery technologies; and the analysis of current and future mobility systems. His research interests are in electrochemical systems with an emphasis on Limetal and Li-ion batteries. Dr. Dufek’s recent work has focused on methods to better understand battery failure modes and how they can be better predicted, quantified, and used for life prediction using limited data. He has published more than 85 peer-reviewed journal articles in electrochemistry, batteries, interface modification, immunoassay development, and corrosion. Dr. Dufek completed his PhD in Analytical Chemistry at the University of Wyoming in 2007. Before joining INL in 2010, he was a postdoctoral research associate at the University of Utah.

60 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org
Student awards—part of the ECS Honors and Awards Program—support the next generation of scientists by expanding opportunities as they progress in their careers. These awards honor student and early career scientists’ dedication and outstanding achievements in their fields of study. SUPPORT THE NEXT GENERATION THROUGH STUDENT AWARDS! Visit www.electrochem.org/student-awards to learn more. Monday, May 29, 2023 1630h BOSTON, MA, USA Sheraton Boston Hotel Join us as we recognize the achievements of distinguished Society award winners and the outstanding past service of volunteers.
NEW MEMBERS NEW MEMBERS NEW MEMBERS NEW MEMBERS
ECS is proud to announce the following new members for October, November, & December 2022 (Members are listed alphabetically by family/last name.)
Members
A
Michael Aubrecht, Hiawassee, GA, USA
B
Matthew Booher, Alpharetta, GA, USA
Michael Boyko, Tempe, AZ, USA
Simon Burkhardt, Ellwangen, BadenWuerttemberg, Germany
C
Jerry Chen, Hsinchu, Taiwan, Taiwan
Yingwen Cheng, Sycamore, IL, USA D
Veena Dhayal, Jaipur, RJ, India
Yanhao Dong, Beijing, Beijing, China
Lauren Fernandez-Vega, San Juan, PR, USA
Robert Fritz, Greenville, SC, USA
G
Jeffrey Gambino, Gresham, OR, USA
Winston Grace, Lauderhill, FL, USA
Alexander Grant, San Francisco, CA, USA
H
Hiroaki Hanafusa, Higashi, Hiroshima, Japan
Anne Hauch, Lyngby, Hovedstaden, Denmark
I
Tomohiro Ishiyama, Tsukuba, Ibaraki, Japan
Swaroop Kagli, Los Angeles, CA, USA
Asif Khan, Brookhaven, GA, USA
Dohyung Kim, Philadelphia, PA, USA
Yong-Hoon Kim, Daejeon, Hoseo, South Korea
Jayne Lee, Hsinchu, Taiwan, Taiwan
Rob Legg, Marlborough, MA, USA
Jin Lim, Dallas, TX, USA
Gerry McCann, Menlo Park, CA, USA
Toshihiro Nakai, Takatsuki, Osaka, Japan
John Oakley, Durham, NC, USA
PSennu Palanichamy, Faridabad, HR, India
Brandon Price, Marietta, GA, USA
RNigel Reuel, Ames, IA, USA
Martin Rozman, Celje, Savinjska, Slovenia
S
Eisuke Sato, Okayama, Okayama, Japan
Michael Shaw, Edwardsville, IL, USA
Andrea Villareal, Katy, TX, USA
Danielle Walkiewicz, Carson City, NV, USA
Ruigang Wang, Tuscaloosa, AL, USA
Y
Jun Yang, Knoxville, TN, USA
Hakan Yildirim, Odense, Syddanmark, Denmark
Ii Yoshihito, Hirakata-shi, Osaka, Japan Z
Qian Zhang, Idaho Falls, ID, USA
Student Members A
Tanya Agarwal, Santa Fe, NM, USA
Kamal Ahammed, Tuscaloosa, AL, USA
Arif Ahmed, Dhubri, AS, India
Grace Temitope Ajayi, London, ON, Canada
Mercy Ajayi, London, ON, Canada
Idil Akyuz, Istanbul, Istanbul, Turkey
Hossein Amiriyarahmadi, London, ON, Canada
Karthik Arunagiri, State College, PA, USA
Marwa Atwa, Stanford, CA, USA
Mirac Ayarci, İstanbul, Pendik, Turkey
Parviz Azimov, Darmstadt, Hessen, Germany
B
Batuhan Bal, Stillwater, OK, USA
Franz Bannert, Garching, Bavaria, Germany
Mustapha Bello, Baton Rouge, LA, USA
Ayush Bhardwaj, Amherst, MA, USA
Supriya Bhaskaran, Magdeburg, Saxony, Germany
Kavin Bhatt, London, England, UK
Ransford Boateng, Detroit, MI, USA
Barbara Bohlen, Wilrijk, Antwerp, Belgium
Moritz Bohn, München, Bavaria, Germany
Jacob Bunting, Oakville, ON, Canada
C
Karthikeyan C., Chennai, TN, India
Arindam Chatterjee, Chennai, TN, India
Frederika Chovancova, Hôrka pri Poprade, Prešov, Slovakia
Yunhan Chuai, Gainesville, FL, USA
Ana Claus, Miami, FL, USA
George Creasey, Ringwood, Hampshire, UK
Jianqiao Cui, Cambridge, MA, USA
D
Raheleh Daneshpour, University Park, PA, USA
Preetisandipta Das, Bhubaneswar, OR, India
Monsuru Dauda, Baton Rouge, LA, USA
Remzi Demirkiran, Gebze, Kocaeli, Turkey
Luke Denoyer, Albuquerque, NM, USA
Dayananda Desagani, Beer Sheva, Southern District, Israel
Quyen Do, Trondheim, Trondelag, Norway
Gulce Dodanli, Darica, Kocaeli, Turkey
Michael Duff, Centennial, CO, USA
Austin Duncan, Canton, GA, USA
E
Ali Ebrahimzadeh Pilehrood, London, ON, Canada
Lukas Esper, Ilmenau, Thuringia, Germany
Cynthia Ezeh, Gainesville, FL, USA
F
Sana Fathima T. K., Chennai, TN, India
G
Alanna Gado, Wallingford, CT, USA
Dipsikha Ganguly, Chennai, TN, India
David García-Bassoco, Ciudad de México, Distrito Federal, México
Anamika Ghosh, Chennai, TN, India
Sourav Ghosh, Chennai, TN, India
Tapan Ghosh, Balasore, OR, India
Theertharaman Govindasamy, Salem, TN, India
Namik Gözüaçık, Gebze, Kocaeli, Turkey
H
Syed Fahad Bin Haque, Dallas, TX, USA
Carla Harzer, Holzkirchen, Bavaria, Germany
Mahmudul Hasan, State College, PA, USA
Catherine Haslam, Ann Arbor, MI, USA
Jan Niklas Hausmann, Berlin, Berlin, Germany
Megan Heath, Trondheim, Sor Trondelag, Norway
Asmaa Heiba, Cairo, Cairo, Egypt
John Hendershot, Baton Rouge, LA, USA
Nguyen Ho, Pullman, WA, USA
F
K
L
M
N
O
VW
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 61
next page)
(continued on
(continued from previous page)
Michael Hoechtl, Vienna, Vienna, Austria
Takumi Ijichi, Sendai-shi, Miyagi, Japan
Jaganathan J., Chennai, TN, India
Saisupriyalakshmi J. S., Chennai, TN, India
Divya Jyothi D, Chennai, TN, India
Annamalai K., Chennai, TN, India
Selva Kumara Raja K., Tirunelveli, TN, India
Sriram K., Thiruvananthapuram, KL, India
Seyedeh Kalantarian, London, ON, Canada
Elif Karakuş, Gebze, Kocaeli, Turkey
Jashanpreet Kaur, Montréal, QC, Canada
Beyza Kaya, Gebze, Kocaeli, Turkey
Hisazaki Kazuma, Nagoya, Aichi, Japan
Fatemeh Keyvani, Waterloo, ON, Canada
Mihyun Kim, Brooklyn, NY, USA
Yeonga Kim, GwangJu, Gyeonggi-do, South Korea
Fatmanur Kocaman Kabil, Gebze, Kocaeli, Turkey
Jana Kupka, Vienna, Vienna, Austria
Evan Kurian, Karaikudi, TN, India
Maximilian Kutter, Bayreuth, Bavaria, Germany
Andy Lazicki, Coralville, IA, USA
Florence Lee, Sheffield, Yorkshire, UK
Kiwoong Lee, Ann Arbor, MI, USA
Maria Isabel Leon Sotelo, Aguascalientes, Aguascalientes, México
Daniel Liao, Ann Arbor, MI, USA
Yuan Liao, St. Andrews Fife, Scotland, UK
Qinglin Lin, College Park, MD, USA
Emma Lord, Whitby, ON, Canada
Kalaiyarasan M., Chennai, TN, India
Kavipriyah M. N., Chennai, TN, India
Debashis Mahato, Chennai, TN, India
William McLeod, Pullman, WA, USA
Justus Metternich, Duisburg, North RhineWestphalia, Germany
Jinhong Min, Ann Arbor, MI, USA
Tingting Mo, London, England, UK
John Morley, London, England, UK
Alexandra Moy, Ann Arbor, MI, USA
Roxana Murgu, St Andrews, Scotland, UK
Khawla Mustafa, Irvine, CA, USA
Esma Mutlu, Istanbul, Sultanbeyli, Turkey
Savithri Mylsamy, Chennai, TN, India
NEW MEMBERS NEW MEMBERS
Manjubaashini Nandhakumar, Coimbatore, TN, India
Niketha Narayana Rao, Chennai, TN, India
Tasya Nasoetion, College Station, TX, USA
Stephanie Oliveras Santos, Madison, WI, USA
Cheranmadevi P., Chennai, TN, India
Sreeraj P., Malappuram, KL, India
Muniyan Palani, Chennai, TN, India
Junghyun Park, Baton Rouge, LA, USA
Sreelakshmi Paruvayakode, Kattangal, KL, India
Abigail Paul, Athens, OH, USA
Ayon Phukan, Bengaluru, KA, India
Pavithra Ponnusamy, Coimbatore, TN, India
Anika Tabassum Promi, Blacksburg, VA, USA
Swathi Puli, Chennai, TN, India
Buwanila Punchihewa, Kansas City, MO, USA
Muhsin Punnoli, Kalpetta, KL, India
Xiaoli Qin, London, ON, Canada
Dhanya R., Chennai, TN, India
Sahaya Michael Hayden R., Nagercoil, TN, India
Anjana Raj Raju, Montréal, QC, Canada
Jason Rakos, Henderson, NV, USA
Kondapalli Tejas Rama Durga Naidu, Chennai, TN, India
Celin Rooth, Chennai, TN, India
S
Maheswari S., Chennai, TN, India
Manju Bharathi S., Chennai, TN, India
MMohamed Arshath S., Chennai, TN, India
Ayisha S. A. F., Ponneri, TN, India
Raghunath Sahoo, Chennai, TN, India
Ram Kishore Sankaralingam, Trichy, TN, India
Parin Shah, Atlanta, GA, USA
Selflando Shehaj, Potsdam, Brandenburg, Germany
Shivam Shekhar, Garhwa, JH, India
Shaghayegh Shoghi, London, ON, Canada
Humera Siddiqui, Darmstadt, Hessen, Germany
Ian Slagle, Atlanta, GA, USA
Jose Solis, Princeton, TX, USA
Shubhang Srivastava, Chennai, TN, India
NWon Joon Suk, Ann Arbor, MI, USA
Quanwen Sun, Idaho Falls, ID, USA
T
Daniel Tague, Dallas, TX, USA
Yukihiro Takahashi, Trondheim, Trondelag, Norway
OYemin Tao, London, England, UK
Vignyatha Tatagari, Lisle, IL, USA
Yafen Tian, Richardson, TX, USA
Stiphany Tieu, State College, PA, USA
Roshaun Titus, Atlanta, GA, USA
Vikrant Trivedi, Chennai, TN, India
Flora Tseng, Ann Arbor, MI, USA
U
Tuğçe Ucun, Gebze, Kocaeli, Turkey
V
Francesco Vanin, London, England, UK
Kelly Varnell, State College, PA, USA
Sudhisha Vasudevan, Chennai, TN, India
Manchala Venkatesh, Chennai, TN, India
W
Hsiao-Hsuan Wan, Gainesville, FL, USA
QJyun-Siang Wang, Tainan City, Tainan, Taiwan
Dominik Weintz, Münster, North RhineWestphalia, Germany
RJingwen Weng, Hefei, Anhui, China
Jacob Wheaton, Ames, IA, USA
Xinyi Wu, London, England, UK
Y
Ezgi Yalçin, Gebze, Kocaeli, Turkey
Guangmeimei Yang, London, England, UK
Sumudu Nimasha Yathramulla
Muhandiramge, Detroit, MI, USA
Huaming Yu, Changsha, Hunan, China
Kunpeng Yu, San Diego, CA, USA
Chuxin Yue, St. Andrews, Scotland, UK
Z
Zhiqiao Zeng, Willington, CT, USA
Ziying Zhan, London, ON, Canada
Liquan Zhang, London, England, UK
Yuchen Zhang, Idaho Falls, ID, USA
Zhenghao Zhu, Knoxville, TN, USA
Berkcan Zülfikar, Gebze, Kocaeli, Turkey
Shangshang Zuo, St Andrews, Scotland, UK
IJ
K
L
P
62 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org

Austria 2 Belgium.................................... 1 Canada.................................. 13 China 3 Denmark................................. 2 Egypt.......................................... 1 Germany 13 India........................................ 48 Israel........................................... 1 Japan 6 Mexico...................................... 2 Norway.................................... 3 Slovakia 2 South Korea......................... 2 Taiwan...................................... 3 Turkey 12 UK............................................... 14 USA.......................................... 82 Austria Belgium Canada China Denmark Egypt Germany India Israel Japan Mexico Norway Slovakia South Korea Taiwan Turkey UK USA BioLogic ....................................................................... 8, 9 ECS Transactions 243rd ECS Meeting 68 El-Cell 21 Gamry ............................................................................... 4 Ion Power 6 IOP back cover Pine Research Instrumentation........................................ 2 Scribner Associates 1 Wiley............................................................................... 46 Advertisers Index The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 63 NEW
NEW
NEW
NEW
New Members by Country Look who joined ECS in the Fourth Quarter of 2022.
MEMBERS
MEMBERS
MEMBERS
MEMBERS
Student Chapter News
ECS Indian Institute of Technology Madras Student Chapter

The student chapter’s December 10, 2022, inaugural event brought 150 participants to the T. T. Jagannathan Auditorium at the Indian Institute of Technology Madras (IIT Madras). The chief guest, Professor A. K. Shukla (Honorary Professor, Indian Institute of Science, Bengaluru) inaugurated the student chapter. Prof. V. Kamakoti (Director, IIT Madras) presided over the event. Prof. R. Gopalan (Adjunct Professor, Department of Metallurgical and Materials Engineering, IIT Madras; former regional director, International Advanced Research Centre for Powder Metallurgy and New Materials [ARCI]) and Dr. Rajendran N (Head, Department of Chemistry, Anna University) attended as well. Faculty Advisors Prof. Kothandaraman Ramanujam, Prof. Ramanathan S, Prof. Raghuram Chetty, and Dr. Raman Vedarajan formally welcomed participants.
To enhance the audience’s electrochemistry knowledge, a workshop was organized to accompany the inaugural event. Prof. Shukla’s Plenary Lecture, “Fueling Future Cars’ Sustainability,” entranced the audience of young researchers and students. The quality of the question and answer session reflected their interest. Prof. Gopalan presented the first keynote lecture, “Electrochemistrydriven Materials for Electric Vehicle Applications.” The talk began with a discussion of basic battery materials and advanced to real-life EV applications. Students were intrigued by this global hot topic and expressed their doubts and concerns in the Q&A session. Prof. Rajendran N delivered the second talk, “Electrochemistry: The Lifeline in Biomaterials Development.” He introduced his research area and presented his work on material synthesis, characterization, and application in body implants—which surprised students.
Based on students’ feedback about the inaugural event, we decided to organize an industry visit to observe electrochemistry processes in person. On December 30, the chapter visited the ARCI IIT Madras research park. The visit’s goal was to view the functioning of fuel cells and their application in power generation and transportation. The students visited facilities that included the synthesis, fabrication, processing, finishing, and characterization labs. The visit encouraged their participation in future chapter activities.

For more information on the chapter, visit the chapter’s website

64 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org STUDENT NEWS
NEWS
STUDENT
The ECS Indian Institute of Technology Madras Student Chapter visits the International Advanced Research Centre for Powder Metallurgy and New Materials (ARCI).
Participants at the December 10, 2022 ECS Indian Institute of Technology Madras Student Chapter Inaugural Event.
International Advanced Research Centre for Powder Metallurgy and New Materials (ARCI) Project Scientist Sundararajan Ramakrishnan explains fuel cells to visiting chapter members
ECS Jawaharlal Nehru University New Delhi Student Chapter
The chapter’s mission is to promote interest in and advancement of the design and application of cutting-edge nanotechnology and nanoscience-based biosensors/sensors, especially diagnostics. Our third event, Sensors for Society, took place on January 12, 2023, in the Jawaharlal Nehru University (JNU) New Delhi Convention Centre committee room. The goal was to provide an ideal academic platform for researchers to disseminate their research and develop technologies and directions in nanobiotechnology, electrochemistry, sensors (gas, bio, and wearable), materials science, and biomedical engineering. The Electrochemical Society (ECS) was the chief funder of the symposium, which was free of charge for attendees.
Prof. Bansi D. Malhotra (former Scientist, National Physical Laboratory and Delhi Technological University), who is known in India as the father of biosensors, gave the opening presentation, on the role of sensors and biosensors in the development of a nation and in a society’s wellbeing. He discussed current technologies for sensors/biosensors in diagnostics, future possibilities, and limitations.
The valuable and enlightening views of Guest of Honor Prof. Satish Chandra Garkoti (Rector-1, JNU, New Delhi) inspired symposium participants. The second Guest of Honor, Ms. Mallika Gope (Director, National Accreditation Board for Testing and Calibration Laboratories [NABL]), delivered a plenary presentation on NABL’s mission and vision. Their goal is to strengthen the accreditation system accepted across the globe by providing high-quality, value-driven services, fostering the APAC/ILAC MRA (Asia Pacific Accreditation Cooperation/International Laboratory Accreditation Cooperation Mutual Recognition Arrangement), empanelling competent assessors, creating awareness among stakeholders, initiating new programs supporting accreditation activities, and pursuing organizational excellence.
The chapter’s faculty advisor, Dr. Pratima Solanki (Assistant Professor, JNU) and Prof. Bijoy K. Kunar (Chairperson, Special Centre for Nanoscience [SCNS], JNU), gave a short introduction on the student chapter.

Keynote speakers discussed the role of electrochemical sensors/ biosensors as promising diagnostic technology that can detect biomarkers in body fluids such as sweat, blood, feces, or urine. They provided insight into the types of electrochemical biosensors and their applications, the importance of gas sensor devices in healthcare, and challenges and future outlook. In “Semiconductor Nanostructure-
based Gas Sensors toward Human Health Monitoring,” Dr. Mrinal Pal (Chief Scientist and Head, Central Glass and Ceramic Research Institute, Kolkata) described developing a breath analyzer for monitoring human health, which can significantly impact society. In “Soaring High: Salivary Nitrite Biosensors,” Dr. Niroj Kumar Sethy (Scientist, Defence Institute of Physiology & Allied Sciences, and Defence Research and Development Organisation) described the development of point-of-care devices using salivary nitrite biosensors for screening people from low altitudes’ preparedness for high altitude ascents. Ms. Prachi Kukreti (Deputy Director, NABL) discussed the process for accrediting testing laboratories. In “Chemiresistive Gas Sensors,” Dr. Akash Katoch (Assistant Professor, Centre for Nanoscience & Nanotechnology, Panjab University, Chandigarh) explained the formation of various core-shell-based nanofiber nanocomposites and their applications in gas sensing. Dr. Debabrata Mishra (Assistant Professor, University of Delhi) reviewed his research on “Development of Spin-based Biosensors using CISS Effect.” A discussion on electrochemical sensors/ biosensors, including challenges, knowledge gaps, and solutions, followed the keynote talks.
We thank the following JNU faculty members who served as oral session chairs:
• Dr. Kavita Arora (Assistant Professor, Advanced Instrumentation Research Facility [AIRF] and School of Computational & Integrative Sciences [SCIS])
• Dr. Poonam Mehta (Assistant Professor, School of Physical Sciences)
• Dr. Priya Gupta (Associate Professor, Atal Bihari Vajpayee School of Management and Entrepreneurship)
• Dr. Ranjana Arya (Assistant Professor, School of Biotechnology)
We also thank Dr. G. B. V. S. Lakshmi (Research Associate, SCNS) and Dr. Tulika Prasad (Assistant Professor, AIRF, SCNS) who evaluated the poster session.
The symposium included presentations by chapter members and other researchers. Six oral talks and six posters reflected the interdisciplinary nature of the event and chapter members’ widespread fields of interest in topics such as gas sensors, biosensors, (continued on next page)
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 65
STUDENT NEWS STUDENT NEWS
Participants at the ECS Jawaharlal Nehru University New Delhi Student Chapter’s third one-day symposium, Sensors for Society, held at the Jawaharlal Nehru University Convention Centre
Photo Credit: Gaurav Sahu, Nano-Bio Laboratory
(continued from previous page)
biomolecules, and theoretical modeling. Tea and lunch breaks and poster sessions allowed researchers from different groups to connect, make new friends, engage in scientific discussions, and network.


Young and budding scientists in the field responded enthusiastically to the fruitful, informative, and encouraging technical sessions. We received reports that the symposium was a satisfactory day of learning, fun, and making new connections. By hosting this event, our student chapter provided a valuable resource for scientists and engineers entering the interdisciplinary field of electrochemical biosensors. We built bridges between different sensor institutes in India and encouraged interdisciplinary dialogue in electrochemistry, sensors, and biosensors.
The chapter and event’s organizing committee convey hearty thanks to JNU Honorable Vice-Chancellor, Professor Santishree D. Pandit, and Rector, Prof. Satish Chandra Garkoti, for obtaining administrative approval to organize Sensors for Society offline on campus. Thanks to the joint efforts of the chapter’s faculty advisor, Dr. Partima Solanki, and chapter members, five invited speaker, and around 100 attendees/researchers from different Indian research groups attended the symposium. We thank the many researchers who participated and made it successful; participants and our partners for supporting our third symposium; and the constant efforts of chapter President Mr. Amit K. Yadav, Secretary Damini Verma, Treasurer Navneet Chaudhary, Vice-President Reena Sajwan, and the whole Nano-Bio Laboratory. We look forward to our next symposium!


ECS Purdue University Student Chapter
After a two-year hiatus, with the lifting of COVID restrictions, the chapter kicked off fall 2022 with a series of in-person seminars based on a common theme, Modeling, Characterization & Analytics in Electrochemical Energy Systems (MoChA). The first invited speaker, Dr. Jordi Cabana (Professor, Department of Chemistry, University of Illinois at Chicago [UIC]; and member, Joint Center for Energy Storage Research, Argonne National Laboratory),
presented “Progress in the Analytical Capability of X-rays to Locate Chemical Phenomena in Battery Materials.” Hosted by the chapter for the entire day, he met Prof. Partha Mukherjee, the chapter’s lead faculty advisor, and interacted with chapter officers and members over lunch. The second invited speaker, Jeffrey Dick (Professor, Purdue Department of Chemistry), spoke on “Probing and Promoting Unique Chemistry in Water Micro and
66 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org STUDENT NEWS STUDENT NEWS
Nano-Bio Laboratory members and chapter officers who organized the Sensors for Society symposium are, from left to right, Secretary Dr. Damini Verma; Dr. G. B. V. S. Lakshmi; Faculty Advisor Dr. Partima Solanki; President Amit K. Yadav; Dr. Awadesh K. Verma; Treasurer Navneet Chaudhary; and Gaurav Sahu
Photo Credit: Dr. Tulika Prasad, Assistant Professor, AIRF and SCNS, JNU
ECS Purdue University Student Chapter lunch with invited speaker Dr. Jordi Cabana and from left to right: Kaustubh G. Naik (member), Susmita Sarkar (Executive Advisor), Anuththara Alujjage (Communications Director), Dr. Avijit Karmakar (member), Sourim Banerjee (Secretary), Dr. Jordi Cabana, Debanjali Chatterjee (President), and Dr. Kingshuk Roy (member).
ECS Jawaharlal Nehru University New Delhi Student Chapter officers are, from left to right, Secretary Damini Verma; Faculty Advisor Partima Solanki; President Amit K. Yadav; and Treasurer Navneet Chaudhary
Photo Credit: Gaurav Sahu, Nano-Bio Laboratory
Prof. Partha P. Mukherjee (ninth from the left); invited speaker, Prof. Jeffrey Dick (eleventh from the left); and the Energy and Transport Sciences Laboratory group.
Nanodroplets.” The chapter is delighted to welcome Prof. Dick as a member of its Advisory Board. The semester’s final invited talk was Brian Tackett (Professor, Purdue Department of Chemical Engineering) on “Modeling Reaction, Convection, and Diffusion for the Electrocatalytic CO2 Reduction Reaction in Fundamental and Applied Systems.”

Chapter President Debanjali Chatterjee and Vice President Aditya Singla took the lead in recruiting students from lab groups working in electrochemistry across various disciplines, such as mechanical engineering, chemical engineering, and chemistry. New members included undergraduates and first-year graduate students.
A successful 2023 chapter event was held in February, the MoChA Poster Symposium , Purdue’s first-ever symposium on


electrochemical sciences and engineering, where undergrads, graduate students, and postdoctoral scholars across several disciplines are invited to showcase their research on electrochemistry in energy storage and conversion. In the spring, the chapter will participate in Purdue NanoDays, a free event organized by Purdue’s Birck Nanotechnology Center to introduce K–12 students, teachers, and parents to the field of nanoscale science through activities and games.
The chapter thanks The Electrochemical Society for providing this impactful platform for scientific outreach and Lead Faculty Advisor Prof. Partha P. Mukherjee for his time and invaluable guidance in conceptualizing these events. The chapter also thanks Interface readers and kindly asks them to follow the chapter’s Twitter handle @EcsPurdue for updates on their latest activities.
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 67 STUDENT NEWS
NEWS
STUDENT
Invited speaker Prof. Brian Tackett presents “Modeling Reaction, Convection, and Diffusion for the Electrocatalytic CO2 Reduction Reaction in Fundamental and Applied Systems.”
ECS Purdue University Student Chapter officers recruit new members over coffee and crêpes. From left to right: Vice President Aditya Singla; newly recruited member, Purdue Chemistry grad student Vanshika Gupta; and President Debanjali Chatterjee.
WE WANT TO HEAR FROM YOU! www.electrochem.org/student-center Send your student chapter news and high resolution photographs to education@electrochem.org

Full issues now available for purchase and download from the ECS Online Store: www.electrochem.org/online-store Volume 111: 243rd Meeting of The Electrochemical Society with SOFC-XVIII 20% ECS Members’ Discount ENHANCE YOUR MEETING EXPERIENCE SOON COMING


The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 69 www.electrochem.org/244 CALL FOR PAPERS Abstract Submission Deadline: April 7, 2023 244th ECS Meeting GOTHENBURG SWEDEN October 8-12, 2023 Swedish Exhibition & Congress Centre
GENERAL INFORMATION
The 244th ECS Meeting takes place in Gothenburg, Sweden, from October 8-12, 2023, at the Swedish Exhibition and Congress Centre. This international conference brings together scientists, engineers, and researchers from academia, industry, and government laboratories to share results and discuss issues on related topics through a variety of formats, including oral presentations, poster sessions, panel discussions, tutorial sessions, ECS Short Courses, professional development workshops, a career fair, and exhibits. The unique blend of electrochemical and solid state science and technology at an ECS meeting provides an opportunity and forum to learn and exchange information on the latest scientific and technical developments in a variety of interdisciplinary areas.
ABSTRACT SUBMISSION
To give an oral or poster presentation at the 244th ECS Meeting, submit an original meeting abstract for consideration, via the ECS website, no later than April 7, 2023. Faxed, emailed, and/or late abstracts are not accepted. Meeting abstracts should explicitly state the work’s objectives, new results, and conclusions or significance. After the submission deadline, symposium organizers evaluate all abstracts for content and relevance to the symposium topic, and schedule accepted submissions as either oral or poster presentations.
Letters of Acceptance/Invitation are sent via email in June 2023, notifying corresponding authors of accepted abstracts, and the date, time, and location of their presentations.
How and when a poster or oral presentation is scheduled is at the symposium organizers’ discretion, regardless of presenters’ requests.
PAPER PRESENTATION
Oral presentations must be in English. LCD projectors and laptops are provided for all oral presentations. Presenting authors MUST bring their presentations on USB flash drives to use with the dedicated laptops located in each technical session room. Speakers requiring additional equipment must make written requests to meetings@electrochem.org at least one month prior to the meeting so appropriate arrangements can be made, subject to availability, and at the author’s expense.
Poster presentations must be displayed in English, on a board approximately 3 feet 10 inches tall by 3 feet 10 inches wide (1.17 meters tall by 1.17 meters wide), labeled with the abstract number and day of presentation as published in the final program.
Digital presenters are required to submit a video of their presentation, and/or a copy of the slide deck or poster that will be made available for on-demand viewing only within the online program through November 4, 2023. Digital presentations are NOT streamed into or out of the onsite session rooms.
MEETING PUBLICATIONS
ECS Meeting Abstracts – All meeting abstracts are published in the ECS Digital Library, copyrighted by ECS, and become ECS’s property upon presentation.
ECS Journals – Authors presenting papers at ECS meetings are encouraged to submit to the Society’s technical journals: Journal of The Electrochemical Society, ECS Journal of Solid State Science and Technology, ECS Advances, and ECS Sensors Plus. Although there is no hard submission deadline, six months from the symposium date is considered sufficient time to revise a paper to meet stricter journal criteria. Author instructions are on the ECS website.
ECS Transactions – Select symposia publish their proceedings in ECS Transactions (ECST). Please check the individual symposia Calls for Papers in this document. Authors presenting in these symposia are welcome to submit to ECST a full-text manuscript based on their presentation. Issues of ECST are available for sale on a pre-order basis, as well as through the ECS Digital Library and ECS Online Store. Review each individual symposium’s listing in this Call for Papers to determine if your symposium is publishing an ECST issue. Visit the ECST website for additional information, including overall guidelines, author and editor instructions, a downloadable manuscript template, and more.
SHORT COURSES
ECS Short Courses provide students and seasoned professionals with indepth education on a wide range of topics in a short, intensive time period. Novices and experts advance their technical expertise and knowledge
through personalized instruction by academic and industry experts. Short Courses require advance registration and may be canceled if course enrollment is under 10 registrants. Learn more at https://www.electrochem. org/short-courses
TECHNICAL EXHIBIT
The 244th ECS Meeting is the right place to exhibit. The Society provides a powerful platform for meeting major new customers while enhancing relationships with current customers from around the world. Coffee and networking breaks, along with evening poster sessions, generate traffic in the exhibit hall.
Your presence at ECS’s leading industry event positions your brand as serious and reliable—and it’s a great way to build buzz for new products! Exhibit opportunities can be combined with sponsorships to suit your marketing needs. Contact sponsorship@electrochem.org for details.
MEETING REGISTRATION
All participants—including authors and invited speakers—are required to pay the appropriate registration fees. Meeting registration information is posted on the ECS website as it becomes available. The deadline for discounted early registration is September 11, 2023.
HOTEL RESERVATIONS
The 244th ECS Meeting takes place at the Swedish Exhibition and Congress Centre. Please refer to the meeting website for the most up-todate information on hotel availability and blocks of rooms where meeting participants receive special rates. The hotel block is open until it sells out or September 11, 2023.

LETTERS OF INVITATION
Letters of Invitation are sent in June 2023 via email to the corresponding authors of all accepted abstracts, notifying them of the date, time, and location of their presentations. Anyone requiring an official Letter of Invitation should email abstracts@electrochem.org. These letters do not imply any financial responsibility on the part of ECS.
BIANNUAL MEETING TRAVEL GRANTS
ECS divisions and sections offer travel grants to assist students, postdoctoral researchers, and young professionals in attending ECS biannual meetings. Applications are available beginning April 7, 2023, at www.electrochem. org/travel-grants. The submission deadline is June 26, 2023. For general travel grant questions, contact travelgrant@electrochem.org
SYMPOSIA FUNDING ASSISTANCE
Additional financial assistance is limited and generally governed by symposium organizers. To inquire if such funding is available, contact the organizers of the symposium in which you are presenting.
SPONSORSHIP OPPORTUNITIES
ECS biannual meetings provide a wonderful opportunity to solidify and strengthen your brand through sponsorship. Sponsor ECS meeting events to heighten your brand visibility and reinforce your position as an industry leader. Companies can choose from a wide array of activities, from symposia to special events, which deliver worldwide recognition as a supporter of electrochemical and solid state research—while enhancing ECS meetings. ECS also offers specific symposium sponsorship. Your sponsorship helps offset symposium travel expenses, registration fees, complimentary proceedings, and/or hosts receptions for invited speakers, researchers, and students. Please contact sponsorship@electrochem.org for further details.
CONTACT INFORMATION
If you have questions or require additional information, contact ECS.
The Electrochemical Society
65 South Main Street, Pennington, NJ, USA 08534-2839
Tel: 1.609.737.1902, fax: 1.609.737.2743
meetings@electrochem.org
www.electrochem.org
70 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org
244th ECS MEETING – SYMPOSIUM TOPICS
A Batteries and Energy Storage
A01 New Approaches and Advances in Electrochemical Energy Systems
A02 Lithium-Ion Batteries
A03 Battery Safety and Failure Modes 4
A04 Next-Generation Batteries
A05 Electrochemical Interfaces in Energy Storage: Theory Meets Experiment
A06 New Developments and Applications of Electrode Binders for Rechargeable Battery and other Electrochemical Systems
A07 Interplay between Temperature and Battery Phenomenon
B Carbon Nanostructures and Devices
B01 Carbon Nanostructures: From Fundamental Studies to Applications and Devices
C Corrosion Science and Technology
C01 Corrosion General Session
C02 Corrosion in Nuclear Energy Systems: From Cradle to Grave 3
C03 Metallic, Organic, Inorganic, and Composite Coatings for Corrosion Protection
C04 Analytical Tools in Corrosion Research
D Dielectric Science and Materials
D01 Semiconductors, Dielectrics, and Metals for Nanoelectronics 20
D02 Plasma and Thermal Processes for Materials Modification, Synthesis, and Processing 5
D03 Advanced 3D Interconnect Technologies and Packaging 2
D04 Water-Energy Nexus Research Relating to Electrochemical Sciences
E Electrochemical/Electroless Deposition
E01 Metal Electrodeposition from Fundamentals to Applications
E02 Electrodeposition of Porous Materials and Materials with Complex Geometries
E03 Electrodeposition of Enhanced Materials: Electrical and Thermal Conductivities
E04 Current Trends in Electrodeposition – An Invited Symposium
F Electrochemical Engineering
F01 Advances in Industrial Electrochemistry and Electrochemical Engineering
F02 Electrochemical Separations and Sustainability 6
F03 Pulse and Reverse Pulse Electrolytic Processes 4: In Honor of E. J. Taylor
F04 Electrochemical Conversion of Biomass 4
G Electronic Materials and Processing
G01 Atomic Layer Deposition and Etching Applications 19
G02 Semiconductor Process Integration 13
H Electronic and Photonic Devices and Systems
H01 State-of-the-Art Program on Compound Semiconductors 66 (SOTAPOCS 66)
H02 Semiconductor Wafer Bonding: Science, Technology, and Applications 17
H03
Low-Dimensional Nanoscale Electronic and Photonic Devices 16
H04 Gallium Nitride and Silicon Carbide Power Technologies 13
H05 Electronic, Thermal, and Electrochemical Properties of Metal Organic Frameworks (MOFs) 3: Technology, Applications, and Emerging Devices
I Fuel Cells, Electrolyzers, and Energy Conversion
I01 Polymer Electrolyte Fuel Cells and Electrolyzers 23 (PEFC&E23)
I02 Photovoltaics for the 21st Century 19: New Materials and Processes
I03 High Temperature Corrosion and Materials Chemistry 15
I04 Ionic and Mixed Conducting Ceramics 14
I05 Photocatalysts, Photoelectrochemical Cells, and Solar Fuels 13
I06 Crosscutting Materials Innovation for Transformational Chemical and Electrochemical Energy Conversion Technologies 5
J Luminescence and Display Materials, Devices, and Processing
J01 Luminescence and Display Materials: Fundamentals and Applications
K Organic and Bioelectrochemistry
K01 Advances in Organic and Biological Electrochemistry
L Physical and Analytical Electrochemistry, Electrocatalysis, and Photoelectrochemistry
L01 Physical and Analytical Electrochemistry, Electrocatalysis, and Photoelectrochemistry General Session
L02 Electrode Processes 14
L03 Advanced Techniques for In Situ Electrochemical Systems 6
L04 Physical and Analytical Electrochemistry in Ionic Liquids 6
L05 Scanning Probe Microscopy 3
L06 Electrochemical Waste Remediation 2
L07 Nanoscale Electrochemistry
L08 Nanostructured Metal Oxides and Polyoxometallate Clusters in Electrocatalysis, Electrochemical Energy Conversion, and Storage
L09 Physical and Electrochemical Processes at Flow Battery Electrodes
L10 Interfacial Analysis for Energy Storage
L11 Everything Voltammetry – Pulsed, Stepped, and Other Waveforms
M Sensors
M01 Recent Advances in Sensors Systems 4
M02 Biosensors, Lab-on-chips, Point-of-care Testing, In Vitro and In Vivo Imaging 2
Z General
Z01 General Student Poster Session
Z02 Electrochemistry in Space 3
Z03 Young Researchers in Europe: A Special Symposium and Workshop
Important Dates and Deadlines
Meeting abstracts submission deadline April 7, 2023
Travel grant applications open April 7, 2023 Notification to corresponding authors of abstract acceptance or rejection
June 12, 2023
Technical program published online June 2023
Meeting registration opens June 2023
ECS Transactions submission site opens June 16, 2023
Travel grant application deadline June 26, 2023
Meeting sponsor and exhibitor deadline (for inclusion in printed materials) July 28, 2023
ECS Transactions submission deadline
July 14, 2023
Travel grant approval notification August 28, 2023
Hotel and early meeting registration deadlines September 11, 2023
Release date for ECS Transactions on or before September 29, 2023
The Electrochemical Society Interface • Spring 2023 • www.electrochem.org 71
ACTIVATE WITH ECS PUBLICATIONS
ECS TOPICAL INTEREST AREAS
Electrochemical
• Batteries and Energy Storage
• Corrosion Science and Technology
• Electrochemical/Electroless Deposition
• Electrochemical Engineering
• Fuel Cells, Electrolyzers, and Energy Conversion
• Organic and Bioelectrochemistry
• Physical and Analytical Electrochemistry, Electrocatalysis, and Photoelectrochemistry
• Sensors (Electrochemical)
Solid State
• Carbon Nanostructures and Devices
• Dielectric Science and Materials
• Electronic Materials and Processing

• Electronic and Photonic Devices and Systems
• Luminescence and Display Materials, Devices, and Processing
• Sensors (Solid State) LEARN MORE
72 The Electrochemical Society Interface • Spring 2023 • www.electrochem.org
2023 ECS INSTITUTIONAL MEMBERS
BENEFACTOR PATRON
BioLogic USA/BioLogic SAS (15*)
Duracell US Operations, Inc. (66)
Gamry Instruments (16)
Gelest Inc. (14)
Hydro-Québec (16)
Pine Research Instrumentation (17)
Energizer Battery (78)
Faraday Technology, Inc. (17)
GE Global Research Center (67)
Lawrence Berkeley National Laboratory (LBNL) (19)
Scribner Associates (27)
Toyota Research Institute of North America (TRINA) (15)
SPONSORING SUSTAINING
BASi (8)
Center for Solar Energy and Hydrogen Research BadenWürttemberg (ZSW) (19)
Center for Synthetic Organic Electrochemistry, University of Utah (2)
Central Electrochemical Research Institute (30)
Corteva Agriscience (1)
Deutsches Zentrum für Luft- und Raumfahrt e.V. (DLR) (15)
Electrosynthesis Company, Inc. (27)
EL-CELL GmbH (9)
Ford Motor Company (9)
GS Yuasa International Ltd. (43)
Honda R&D Co. (16)
Medtronic, Inc. (43)
Nissan Motor Co., Ltd. (16)
Pacific Northwest National Laboratory (PNNL) (4)
Panasonic Energy Corporation (28)
Permascand AB (20)
Plug Power, Inc. (2)
Teledyne Energy Systems, Inc. (24)
UL Research Institutes (2)
Advanced Cell Engineering (2)
Cummins, Inc. (5)
Current Chemicals (1)
General Motors Holdings LLC (71)
Giner, Inc. (37)
Ion Power, Inc. (9)
Kanto Chemical Co., Inc. (11)
Los Alamos National Laboratory (LANL) (15)
Metrohm USA, Inc. (9)
Microsoft Corporation (6)
Occidental Chemical Corporation (81)
Sandia National Labs (47)
Sherwin-Williams (2)
Spectro Inlets ApS (1)
Technic, Inc. (27)
United Mineral & Chemical Corporation (2)
Western Digital GK (9)
Westlake Corporation (28)
Yeager Center for Electrochemical Sciences at CWRU (25)
Please help us continue the vital work of ECS by joining as an institutional member today. To renew, join, or discuss institutional membership options please contact Anna Olsen, Senior Manager, ECS Corporate Programs, anna.olsen@electrochem.org.
*Years of membership as of 6 January 2023
ECS, a prestigious nonprofit professional society, has led the world in electrochemistry, solid state science and technology and allied subjects since 1902, providing a rigorous and high-quality home for the whole community.
ECS is dedicated to moving science forward by empowering researchers globally to leave their mark on science. The Society connects a diverse and representative constituency of members and nonmembers to accelerate scientific discovery, facilitate the engagement of an inclusive network, and champion the dissemination of research to support a sustainable future.
For more information on becoming a member, or publishing in ECS publications, visit electrochem.org













 by Rashmi Jha
by Rashmi Jha















































































 by M. E. Orazem and Kevin J. Otto
by M. E. Orazem and Kevin J. Otto

















































































