
EXECUTIVE COUNCIL
RUTH-ANNE RICHTER BSc (Hon), DVM, MS FAEP COUNCIL PAST PRESIDENT rrichter@brandonequine.com

ADAM CAYOT DVM adamcayot@hotmail.com


RUTH-ANNE RICHTER BSc (Hon), DVM, MS FAEP COUNCIL PAST PRESIDENT rrichter@brandonequine.com

ADAM CAYOT DVM adamcayot@hotmail.com
In our third issue of The Practitioner this year, we’re excited to focus on important topics such as how to treat joint disease, ultrasound techniques of the equine carpal canal, and biosecurity lessons.
I would like to remind all our readers that our 18th Annual Promoting Excellence Symposium (PES) is fast approaching! Don’t miss your chance to learn from Dr. Jean-Marie Denoix, the world’s foremost equine musculoskeletal system anatomist.

Dr. Denoix will be leading our ultrasound wet lab, featuring six anatomical stations, which will do everything from teaching you how to use ultrasound equipment most effectively to honing your advanced diagnostic sonography skills. You will get ample opportunity to practice your skills on real horses while supervised by our esteemed experts who will provide helpful tips and feedback.
SALLY ANNE DENOTTA DVM, PhD s.denotta@ufl.edu
COREY MILLER DVM, MS, DACT cmiller@emcocala.com


PES will be held Oct. 19-22, 2023, in West Palm Beach, FL, at the Hilton West Palm Beach. This excellent conference offers veterinarians 41 RACE-approved CE credits and features Florida’s mandatory three-hour course, Florida Laws & Rules and Dispensing Legend Drugs.
I hope to see you there!
Best wishes,
ANNE L. MORETTA VMD, MS, CVSMT, CVA maroche1@aol.com

Armon Blair, DVM FAEP Council President Ocala Equine Hospital abeqdoc@gmail.com
JACQUELINE S. SHELLOW DVM, MS FVMA PRESIDENT jackie@shellow.com

FAEP Mission: Support our members’ professional development and educate them on issues affecting the equine industry as well as methods for improving equine welfare in Florida.
Opinions and statements expressed in The Practitioner reflect the views of the contributors and do not represent the official policy of the Florida Association of Equine Practitioners (FAEP) or the Florida Veterinary Medical Association (FVMA), unless so stated.

The FAEP is the equine-exclusive division of the FVMA. The FAEP is focused on supporting the professional development of its members and providing them with world-class, equine-exclusive continuing education through two annual conferences.
■ Discounts on Continuing Education
Members can access online courses and conferences for affordable continuing education opportunities. Our yearly equine events, the Ocala Equine Conference (OEC) and the Promoting Excellence Symposium (PES) enhance attendees' skills and knowledge through lectures and hands-on wet labs. These conferences also provide a platform for networking with colleagues and industry partners.
■ Complimentary Legal Consultations
Members have always been able to ask our legal team questions on practicing veterinary medicine, pharmacy law, and veterinary board relations. Our legal benefits have expanded in 2023 to include new areas of law such as labor and employment law and civil litigation.
■ Discounts on Services and Goods
FAEP members have access to exclusive discounts on merchant services, professional services , digital marketing tools, and travel and entertainment. These include:
■ Clover Merchant Services for electronic payments.
■ CareCredit for client financing and payment options.
■ VetSocial for curated, equine-exclusive social media marketing support

■ Working Advantage for deals on everything from theme park tickets to rental cars, movie tickets, streaming services, and more.
**FAEP Bylaws state: Any veterinarian who has been (a) an active member of the Association or any other state veterinary association for at least the past 15 years, (b) has reached the age of 65, (c) and who may be engaged in the practice of veterinary medicine on an average of less than a total of ten (10) hours per week. Retired members shall have all the privileges of full membership.
Joint disease is common in the equine athlete and osteoarthritis (OA) remains the leading cause of lameness in the equine industry, affecting approximately 60% of the equine population. Posttraumatic OA (PTOA), which occurs secondary to acute or chronic joint trauma, is likely the most common type of OA in the horse. Trauma can be caused by such things as repetitive loading, intraarticular fractures, osteochondritis dissecans (OCD), and soft tissue injuries (e.g., meniscal tears, collateral ligament tears). Secondary to trauma, global posttraumatic inflammation of the articular environment is caused by increased synthesis of catabolic cytokines and degradative enzymes that lead to unregulated, progressive degeneration of the articular cartilage.¹,²
Currently, there are no effective disease-modifying drugs that halt or reverse OA in the horse with treatment regimens being aimed at reducing the clinical signs associated with OA. Unfortunately, joint disease often leads to early retirement or euthanasia.³
The two main goals of treatment for joint disease are:
1. decrease pain and lameness
2. minimize the progression of joint degeneration
Standard medical treatments for OA have included systemic therapy with non-steroidal anti-inflammatory drugs (NSAIDs) and other medications aimed at supporting joint health (e.g., polysulfated glycosaminoglycans (PSGAGs), hyaluronic acid); intra-articular therapy with corticosteroids, hyaluronic acid, and PSGAGs; and controlled exercise. During the past decade, significant research and clinical focus has been on the
use of orthobiologics in the horse with the goal of limiting further degeneration by -orthobiologics used in equine joint disease. Other newer treatments include intra- articular polyacrylamide hydrogels. Many horses with joint disease can also be treated surgically. Options for surgical intervention may include stabilization of intra-articular fractures, removal of osteochondral fragments, cartilage repair, facilitated ankylosis, and arthrodesis.
Intra-articular corticosteroids have been the mainstay of joint disease treatment for many decades and, despite some of the negative connotations associated with this class of drugs, can be very effective in treating joint disease in many horses. Corticosteroids act as potent anti- inflammatories by inhibiting the arachidonic acid cascade by blocking phospholipase A 2 .
Figure 1. Joint disease can be treated with a variety of intra-articular injections. A) Corticosteroids, such as triamcinolone, are commonly used to treat pain and inflammation. B) Polyacrylamide hydrogels, such as Noltrex®Vet, work as synthetic joint lubricants. More recently, orthobiologics are used to treat joint disease due to their anti-inflammatory and pro-healing properties. These include such products as C) autologous conditioned serum (IRAP II®, Arthrex) which is prepared following incubation of blood overnight in a tube with borosilicate beads leading to increased concentrations of interleukin-1 receptor antagonist (IL-1Ra) amongst other growth factors and cytokines, D) autologous protein solution (Pro-Stride®, Zoetis) which is generated patient-side following two centrifugation steps yielding a final product that contains concentrated platelets, IL-1Ra, and other growth factors and cytokines, and E) alpha-2 macroglobulin (Alpha2Eq, Astaria Global), a proteinase inhibitor that decreases extra-cellular matrix degradation. Image courtesy of Dr. Kyla Ortved.

They have been shown to decrease expression and synthesis of IL-1β, TNF-α, MMP-3, and MMP-13 in cartilage, thereby impeding the degradative processes in the joint.⁴ Currently, the three main corticosteroids used by equine practitioners include methylprednisolone acetate (Depo-Medrol), triamcinolone acetonide (Vetalog), and betamethasone acetate (Betavet). Previous studies have shown that while all formulations have beneficial effects on lameness, triamcinolone also has some protective effects on cartilage, while methylprednisolone actually has some detrimental effects on chondrocytes and the ECM.5–7 These findings led to the AAEP suggestion that triamcinolone be used in high-motion joints, while methylprednisolone be reserved for low-motion joints.
Despite the beneficial clinical effects of corticosteroids, a recent trend away from them is notable, likely due to a combination of potential detrimental effects on the joint and stricter withdrawal periods imposed by racing authorities. Additionally, although the reported incidence of corticosteroid-induced laminitis in the literature is quite low (0.15-0.5%),⁸,⁹, there are rising concerns about laminitis post-corticosteroid administration in horses with pre-existing endocrinopathies such as pituitary pars intermedia dysfunction (PPID; Equine Cushing’s Disease) or insulin dysregulation. Further research is needed in this rapidly growing population of horses.
Hyaluronic acid (HA), a disaccharide of D-glucuronic acid and N-acetyl-D-glucosamine, is a major component of synovial fluid and aggrecan. When administered intra-articularly, HA plays a role in lubrication and has anti-inflammatory effects, particularly on white blood cells, in the joint. It is also believed to increase endogenous production of HA by synoviocytes. High molecular weight HA is reported to be more effective;, however, it is also associated with higher costs. Hyaluronic acid is likely more effective for treatment of mild synovitis or capsulitis versus more severe cases of OA.¹⁰ Combination therapy of HA and corticosteroids is common, although a recent study found that lameness was decreased more in horses treated with triamincolone alone compared to HA + triamcinolone.¹¹ At this time, the jury is still out on the effectiveness of combination therapy.
Polysulfated polysaccharides, including polysulfated glycosaminoglycan (PSGAG; Adequan) and pentosan polysulfate, have been shown to inhibit catabolic cytokines and degradative enzymes in the joint.¹² Both products can be administered intra-muscularly (IM) or intra- articularly (IA);, however, only Adequan is FDA-approved, and this approval is only limited to IM administration. Intramuscular PSGAG administered every four days for seven treatments have been
shown to slightly decrease lameness in an experimental OA study when compared to placebo;, although, when the study was repeated with IA PSGAG administered once a week for two weeks more significant improvements were noted.¹³ It should be noted that there is evidence that IA administration of Adequan lowers the bacterial inoculation dose of Staphylococcus such that co- administration of amikacin (125mg) is recommended.¹⁴ There is little evidence in the literature to support the use of pentosan polysulfate;, although, anecdotal reports are favorable.

Bisphosphonates are a class of drug that inhibit osteoclastic activity in bone, thereby slowing bone loss. Currently, clodronate (OsPhos) and tiludronate (Tildren) are available for use in the horse. Although neither drug is approved for use in horses with OA, several studies have suggested beneficial effects in horses with distal tarsal OA and axial skeleton OA.¹⁵ ,¹⁶ Because bisphosphonates have a remarkably long half-life in bone (up to 10 years),¹⁷ the long-term effect of osteoclastic inhibition requires careful consideration.
Polyacrylamide hydrogels (PAAG) have been recently introduced for use in synovial joints. Polyacrylamide hydrogels are synthetic, non-degradable, and biocompatible injectable gels that can be used for viscosupplementation in joints. They have been shown to integrate into the host’s soft tissues. Specifically, PAAGs integrate into the sub-synovial layer of rabbit and horse joints and persist for at least 2 years.¹⁸ It is proposed that sub-synovial integration alters the biologic and/or mechanical properties of the synovium, thereby, supporting joint health and decreasing pain. Further studies are necessary to fully elucidate the mechanism of action of PAAGs and to determine if there are any long-term side effects associated with the non-degradable substance. Preliminary studies in the horse demonstrated analgesic benefits in OA joints up to 2 years post injection.¹⁹ Currently, Noltrex (4% PAAG) and Arthramid (2.5% PAAG) are approved for use in the US.
Platelet rich plasma (PRP) is defined as a volume of plasma with a platelet count above that of whole blood, although the fold increase in platelet count is highly variable between different products. PRP can be prepared patient- side following centrifugation or gravity filtration of autologous blood as platelets are smaller and less dense than RBCs and WBCs. Several commercial systems are available including Arthrex ACP®
(Arthrex, Naples, FL), GPS® III (Zimmer Biomet, Warsaw, IN), Harvest® SmartPrep® (Terumo BCT, Lakewood, CO), Restigen PRP® (Owl Manor Veterinary, Warsaw, IN), and others. There is great variability in platelet and WBC concentration in the final products. The therapeutic effect of PRP is in large part due to degranulation of platelet α-granules that release growth factors including PDGF, TGF-β, FGF, VEGF, and EGF which modulate the healing response. PRP also promotes healing by enhancing cell migration, proliferation and differentiation, improving matrix synthesis, and stimulating angiogenesis.²⁰ Studies have shown that PRP is safe to inject into equine joints, with a minimal, transient increase in nucleated cell count noted following injection.²¹ PRP has also been shown to improve lameness scores in horses with naturally occurring fetlock arthritis.²²
Autologous conditioned serum (ACS) is another blood-based product that contains concentrated interleukin-1 receptor antagonist (IL-1Ra or IRAP) in addition to other cytokines and cytokines. IL-1Ra is an endogenous protein produced by immune cells, mainly monocytes, and is a competitive antagonist of interleukin-1 (IL-1), a central mediator of inflammation and degradation in joints. IRAP II (Arthrex Vet Systems) and Orthokine (Dechra Veterinary Products) are two commercially available products that rely on a 24hour incubation of autologous blood in a specialized syringe that works to concentrate IL-1Ra. Following incubation, the conditioned serum is collected and used for IA injections with the excess being stored -20ºC for future injections. A common treatment regimen includes injection of the affected joint every
Collection of bone marrow from the sternum in a standing, sedated horse.seven - ten days for three to five treatments. Few studies have been performed evaluating the effect of ACS; however, Frisbie et al. have demonstrated improved clinical outcomes in horses with experimentally induced OA treated with ACS.²³
Autologous protein solution (APS) is a newer patient-side product that contains concentrated platelets and increased concentrations of IL-1Ra. This product, marketed as ProStride, was formerly distributed by Owl Manor Veterinary but was recently acquired by Zoetis. The combination of growth factors from platelets and IL-1Ra is suggested to have a synergistic effect in the joint. In addition, the system does not require incubation and can be used patient-side. One study has shown improved lameness scores in osteoarthritic horses treated with Pro-Stride.²⁴
Alpha-2-macroglobulin (A2M) is an endogenous protease inhibitor that can be concentrated during processing of autologous blood. A2M binds several catabolic cytokines, including IL-1β and TNF-α, and appears to neutralize MMP activity. Although there are currently no studies evaluating the clinical effect of A2M in the horse, anecdotal reports are quite favorable. Additionally, A2M has been shown to protect the ECM in rats with anterior cruciate ligament transection.²⁵
Equine amnion-based products are available for treating joints
(AniCell Biotech). Amnion is collected and decellularized such that an off-the-shelf bioscaffold is available. At this time, support for the use of amnion in equine joint disease is mainly anecdotal.


Stem Cells
Stem cells are undifferentiated cells that are capable of selfrenewal and able to differentiate into different cell types (potency). Adult-derived mesenchymal stem cells (MSCs) are used most commonly in equine medicine and are mainly obtained from bone marrow and adipose tissue. Following collection, MSCs can be immediately concentrated for subsequent use, or they can be culture- expanded in a lab. The therapeutic effect of MSCs is mainly attributed to their
 Culture expansion of autologous mesenchymal stem cells (MSCs). Image courtesy of Dr. Kyla Ortved
Microscopic image of MSCs showing their typical spindle-cell shape. Image courtesy of Dr. Kyla Ortved
Culture expansion of autologous mesenchymal stem cells (MSCs). Image courtesy of Dr. Kyla Ortved
Microscopic image of MSCs showing their typical spindle-cell shape. Image courtesy of Dr. Kyla Ortved
immunomodulatory effects and ability to recruit endogenous progenitor cells, while engraftment and differentiation play a more minor role. Intra-articular MSCs have been shown to improve healing and alleviate clinical signs associated with OA and appear to be quite promising in horses with meniscal tears.²⁶ -²⁸ The number of cells for injection depends on the size of the joint, generally ranging from 10-50 million cells/ joint. Injections can be repeated every four weeks for three to five treatments. Several equine studies have also reported the resurfacing of full-thickness cartilage lesions with MSCs with variable success.²⁹-³¹ This can be performed arthroscopically using a self-polymerizing fibrin glue to maintain the MSCs in the grafted defect.
Many horses with joint disease can be treated with surgery, either alone or in combination with medical treatment. Surgical treatment is strongly indicated in the treatment of articular fractures with the goal of reconstruction and stabilization of the articular surface. Lag screw fixation is particularly useful for articular fracture repair and can significantly improve longterm outcomes by preserving the health of the joint. Horses with osteochondral fragments, including chip fractures and

OCD, will also benefit from surgical intervention. Arthroscopic removal of loose cartilage flaps and free-floating fragments decrease the negative mechanical and biologic stimulus in the joint thereby restoring homeostasis and limiting further degradation. In some cases, focal chondral lesions can be repaired with advanced cartilage repair techniques including osteochondral transplantation (mosaicplasty),³² autologous chondrocyte implantation (ACI),³³ or MSC-based grafting.²⁹ In more severely affected joints that are no longer responsive to medical therapy, fusion of the joint may become the goal. Facilitated ankylosis of the distal tarsal joints using ethyl alcohol injections or transarticular drilling has been reported with fair success rates for return to soundness.³⁴,³⁵ Finally, arthrodesis can be an excellent option for either return to athletic work or elimination of pain depending on the joint. Arthrodesis of lower motion joints such as the distal tarsal joints, carpometacarpal joint, or proximal interphalangeal joint can yield horses capable of returning to their previous athletic use. Arthrodesis of higher motion joints such as the fetlock or carpus can provide longterm comfort to horses, but a mechanical gait deficit will remain.
Physical therapies including extracorporeal shock wave (ESWT), cryotherapy, therapeutic shoeing, therapeutic ultrasound,
Figure 2. A) Lateral-medial radiograph of a horse with severe osteoarthritis of the proximal interphalangeal joint (pastern joint). B) Treatment for this horse consisted of a pastern arthrodesis with a dorsal locking compression plate and transarticular screws. The arthrodesis led to fusion of the joint and long-term soundness.therapeutic laser therapy, and pulsed electromagnetic field (PEMF) may benefit horses with joint disease;, however, evidence for their use is scant. Studies have demonstrated improvement in clinical lameness in horses treated with ESWT following induction of OA.¹³ Controlled exercise is perhaps one of the most important considerations for horses with joint disease. Like humans, horses with OA benefit from movement versus stall rest and confinement. Hopefully as the field of equine physical therapy and rehabilitation expands, we will have more evidence-based medicine on which to make recommendations.
1. Mankin HJ. The response of articular cartilage to mechanical injury. J bone Jt surgeryAmerican Vol. 1982;64(3):460-466. doi:10.1007/978-1-4471-5451-8_96
2. Lieberthal J, Sambamurthy N, Scanzello CR. Inflammation in joint injury and post- traumatic osteoarthritis. Osteoarthr Cartil. 2015;23(11):1825-1834. doi:10.1016/j. joca.2015.08.015
3. Oke SL, Mcilwraith CW. Review of the Economic Impact of Osteoarthritis and Oral Joint-Health Supplements in Horses. Am Asocciation Equine Pract. 2010;56:12-16. Accessed February 19, 2019. https://www.cabdirect.org/ cabdirect/abstract/20113042298
4. Garvican ER, Vaughan-Thomas A, Redmond C, Gabriel N, Clegg PD. MMP-mediated collagen breakdown induced by activated protein C in equine cartilage is reduced by corticosteroids. J Orthop Res. 2010;28(3):370-378. doi:10.1002/JOR.21001
5. Frisbie DD, Kawcak CE, Baxter GM, et al. Effects of 6alphamethylprednisolone acetate on an equine osteochondral fragment exercise model. Am J Vet Res. 1998;59(12):16191628.
6. Frisbie DD, Kawcak CE, Trotter GW, Powers BE, Walton RM, McIlwraith CW. Effects of triamcinolone acetonide on an in vivo equine osteochondral fragment exercise model. Equine Vet J. 1997;29:349-359.
7. FOLAND JW, MCILWRAITH CW, TROTTER GW, POWERS BE, LAMAR CH. Effect of betamethasone and exercise on equine carpal joints with osteochondral fragments. Vet Surg. 1994;23(5):369-376. doi:10.1111/ J.1532-950X.1994.TB00497.X
8. Bathe AP. The corticosteroid laminitis story: 3. The clinician’s viewpoint. Equine Vet J. 2007;39(1):12-13. doi:10.2746/042516407X165801
9. McCluskey MJ, Kavenagh PB. Clinical use of triamcinolone acetonide in the horse (205 cases) and the incidence of glucocorticoid-induced laminitis associated with its use. Equine Vet Educ. 2004;16(2):86-89. doi:10.1111/J.2042-3292.2004.TB00272.X
10. Frisbie DD, Kawcak CE, McIlwraith CW, Werpy NM. Evaluation of polysulfated glycosaminoglycan or sodium hyaluronan administered intra-articularly for treatment of horses with experimentally induced osteoarthritis. Am J Vet Res. 2009;70(2):203-209.
11. de Grauw JC, Visser-Meijer MC, Lashley F, Meeus P, van Weeren PR. Intra-articular treatment with triamcinolone compared with triamcinolone with hyaluronate: A randomised open-label multicentre clinical trial in 80 lame horses. Equine Vet J. 2016;48(2):152-158. doi:10.1111/ EVJ.12383
12. Todhunter RJ, Lust G. Polysulfated glycosaminoglycan in the treatment of osteoarthritis. J Am Vet Med Assoc. 1994;204(8):1245-1251. Accessed July 30, 2022. https:// pubmed.ncbi.nlm.nih.gov/8014098/
13. Frisbie DD, Kawcak CE, McIlwraith CW. Evaluation of the effect of extracorporeal shock wave treatment on experimentally induced osteoarthritis in middle carpal joints of horses. Am J Vet Res. 2009;70(4):449-454.
14. Gustafson S, McIlwraith C, Jones R. Comparison of the effect of polysulfated glycosaminoglycan, corticosteroids, and sodium hyaluronate in the potentiation of a subinfective dose of Staphylococcus aureus in the midcarpal joint of horses. Am J Vet Res. 1989;50(12):2014-2017.
15. Coudry V, Thibaud D, Riccio B, Audigié F, Didierlaurent D, Denoix J-M. Efficacy of tiludronate in the treatment of horses with signs of pain associated with osteoarthritic lesions of the thoracolumbar vertebral column. Am J Vet Res. 2007;68(3):329-337. doi:10.2460/ajvr.68.3.329
16. Gough MR, Thibaud D, Smith RKW. Tiludronate infusion in the treatment of bone spavin: a double blind placebo-controlled trial. Equine Vet J. 2010;42(5):381-387. doi:10.1111/j.2042-3306.2010.00120.x
17. Lin JH. Bisphosphonates: a review of their pharmacokinetic properties. Bone. 1996;18(2):75-85. Accessed July 16, 2019. http://www.ncbi.nlm.nih.gov/pubmed/8833200
18. Christensen L, Camitz L, Illigen KE, Hansen M, Sarvaa R, Conaghan PG. Synovial incorporation of polyacrylamide hydrogel after injection into normal and osteoarthritic animal joints. Osteoarthr Cartil. 2016;24(11):1999-2002. doi:10.1016/J.JOCA.2016.07.007
19. Tnibar A, Schougaard H, Camitz L, et al. An international multi-centre prospective study on the efficacy of an intraarticular polyacrylamide hydrogel in horses with osteoarthritis: a 24 months follow-up. Acta Vet Scand. 2015;57(1). doi:10.1186/S13028-015-0110-6
20. Textor J. Autologous Biologic Treatment for Equine Musculoskeletal Injuries: Platelet- Rich Plasma and IL-1 Receptor Antagonist Protein. Vet Clin North Am Equine Pract. 2011;27(2):275-298. doi:10.1016/j.cveq.2011.05.001
21. Textor JA, Tablin F. Intra-articular use of a platelet-rich product in normal horses: clinical signs and cytologic responses. Vet Surg. 2013;42(5):499-510. doi:10.1111/j.1532950X.2013.12015.x
22. Broeckx S, Zimmerman M, Crocetti S, et al. Regenerative Therapies for Equine Degenerative Joint Disease: A Preliminary Study. Kerkis I, ed. PLoS One. 2014;9(1):e85917. doi:10.1371/journal.pone.0085917
23. Frisbie DD, Kawcak CE, Werpy NM, Park RD, McIlwraith CW. Clinical, biochemical, and histologic effects of intraarticular administration of autologous conditioned serum in horses with experimentally induced osteoarthritis. Am J Vet Res. 2007;68(3):290-296.
24. Bertone AL, Ishihara A, Zekas LJ, et al. Evaluation of a single intra-articular injection of autologous protein solution for treatment of osteoarthritis in horses. Am J Vet Res. 2014;75(2):141-151. doi:10.2460/ajvr.75.2.141
25. Zhang Y, Wei X, Browning S, Scuderi G, Hanna LS, Wei L. Targeted designed variants of alpha-2-macroglobulin (A2M) attenuate cartilage degeneration in a rat model of osteoarthritis induced by anterior cruciate ligament transection. Arthritis Res Ther. 2017;19(1). doi:10.1186/ S13075-017-1363-4
26. McIlwraith CW, Frisbie DD, Rodkey WG, et al. Evaluation of intra-articular mesenchymal stem cells to augment healing of microfractured chondral defects. Arthroscopy. 2011;27(11):1552-1561. doi:10.1016/j.arthro.2011.06.002
27. Frisbie DD, Kisiday JD, Kawcak CE, Werpy NM, McIlwraith CW. Evaluation of adipose- derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res. 2009;27(12):1675-1680. doi:10.1002/jor.20933 [doi]
28. Ferris DJ, Frisbie DD, Kisiday JD, et al. Clinical outcome after intra-articular administration of bone marrow derived mesenchymal stem cells in 33 horses with stifle injury. Vet Surg. 2014;43(3):255-265. doi:10.1111/j.1532950X.2014.12100.x [doi]
29. Frisbie DD, McCarthy HE, Archer CW, Barrett MF, McIlwraith CW. Evaluation of articular cartilage progenitor cells for the repair of articular defects in an equine model. J Bone Joint Surg Am. 2015;97(6):484-493. doi:10.2106/JBJS.N.00404
30. Wilke MM, Nydam D V., Nixon AJ. Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. J Orthop Res. 2007;25(7):913-925. doi:10.1002/jor.20382
31. Fortier LA, Potter HG, Rickey EJ, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. JBJS . 2010;92:1927-1937.
32. Bodo G, Hangody L, Modis L, Hurtig M. Autologous osteochondral grafting (mosaic arthroplasty) for treatment of subchondral cystic lesions in the equine stifle and fetlock joints. Vet Surg VS Off J Am Coll Vet Surg. 2004;33(6):588596. doi:10.1111/j.1532- 950X.2004.04096.x
33. Ortved KF, Begum L, Mohammed HO, Nixon AJ. Implantation of rAAV5-IGF-I transduced autologous chondrocytes improves cartilage repair in full-thickness defects in the equine model. Mol Ther. Published online October 14, 2014. doi:10.1038/mt.2014.198 [doi]
34. Carmalt JL, Bell CD, Panizzi L, Wolker RRE, Lanovaz JL, Wilson DG. Alcohol- facilitated ankylosis of the distal intertarsal and tarsometatarsal joints in horses with osteoarthritis. J Am Vet Med Assoc. 2012;240(2):199-204.
35. Adkins AR, Yovich J V, Steel CM. Surgical arthrodesis of distal tarsal joints in 17 horses clinically affected with osteoarthritis. Aust Vet J. 2001;79(1):26-29.
Dr. Kyla Ortved is an Assistant Professor of Large Animal Surgery at New Bolton Center, University of Pennsylvania in Kennett Square, PA. She received her DVM degree from the University of Guelph in 2006 and completed her large animal surgical residency training at Cornell University in 2010. Kyla became boarded with the American College of Veterinary Surgeons in 2011. Following her residency, Kyla went on to obtain a PhD in gene therapy for equine cartilage repair at Cornell. In February 2016, Kyla became boarded with the American College of Veterinary Sports Medicine and Rehabilitation. She joined the large animal surgery faculty at New Bolton Center in 2016 as an equine orthopedic surgeon and was named the Jacques Jenny Endowed Term Chair of Orthopedic Surgery in 2019. Her research program focuses on understanding the pathophysiology of equine osteoarthritis and developing gene and cellbased therapies to improve cartilage repair and prevent osteoarthritis.

Kyla Ortved, DVM, PhD, DACVS, DACVSMR
better.
Our updated three-part course satisfies Florida’s mandatory continuing education (CE) requirement for license renewal of no less than one hour of CE in the area of dispensing legend drugs and two hours of CE in the area of the laws and rules governing the practice of veterinary medicine.
KEY TOPICS include:
• The disciplinary process and guidelines
• Record-keeping requirements
• Appropriate drug dispensing practices

• DEA regulations
• Common causes for disciplinary action and how to avoid them
• Recent statutory, regulatory, and case law developments
Note: This course is approved by the Florida Board of Veterinary Medicine, Provider #0001682.
Non-member: $149.00

Proven

People


Our Purina PhD Nutritionists tackle problems using science. And our love of horses keeps us at it until we get it right. Even with our most established feeds, we keep innovating. Even when it takes years of research, we don’t stop until it’s right. We’re dedicated to the scientific method, but it can’t capture the feeling of seeing a horse reach their full potential. It takes science and love to help your horse live their best life.

Put our research to the test at equinevetnutrition.com

Effusion in the carpal sheath is an indication for ultrasound examination of the carpal canal. Septic tenosynovitis, superficial digital flexor (SDF) and deep digital flexor (DDF) tendinopathies, desmopathy of the accessory ligament of the superficial digital flexor tendon (AL-SDFT), and distal radial osteochondromas can easily be diagnosed with ultrasound. Ultrasound of this area is not done frequently, but understanding the anatomy makes this a very straightforward procedure. This article will illustrate the anatomy, ultrasound examination technique, and normal ultrasound appearance of the structure in the carpal canal. Note that medical and proximal are to the left in all ultrasound images.
The carpal canal is formed by the proximal aspect of the ALSDFT medially, the accessory carpal bone and associated ligaments laterally, the palmar ligament dorsally, and the palmar carpal retinaculum and palmar carpal fascia on the palmar surface. The carpal sheath is contained within the carpal canal and extends distally to the AL-DDFT in the proximal metacarpus. Structures within the carpal sheath include the SDF, AL-SDFT, DDFT, flexor carpi radialis (FCR), and the median artery and nerve. The cephalic vein, medial vein, and distal radial vessels are superficial to the palmar carpal retinaculum.
The SDF muscle originates on the medial epicondyle of the humerus, the olecranon, and the caudal surface of the radius. The musculotendinous junction is near the level of the chestnut. Distal to the accessory carpal bone, the SDF becomes palmar in orientation and continues to its
insertions on the medial and lateral aspect of P2. When examining the SDF in the proximal metacarpal area it is important to continue proximally to evaluate the portion in the carpal canal.
The AL-SDFT (also known as the superior check ligament) originates from the caudomedial aspect of the radius near the level of the chestnut. It is trapezoidal in shape and thins before it fuses with the SDF tendon. It travels in a proximodorsal to distopalmar direction.
The DDF muscle originates on the medial epicondyle of the humerus, the olecranon, and the caudal surface of the radius. The radial head of the DDF muscle is deep, just caudal to
Suzan Oakley | DVM, Dipl ACVSMR, Dipl ABVP (Equine), Certified Member, ISELP Wellington Equine Sports Medicinethe radius. It is more echogenic than the adjacent muscle and has fewer variations in echogenicity. The ulnar and humeral muscle bellies of the DDF are superficial to the radial head and are more lateral.
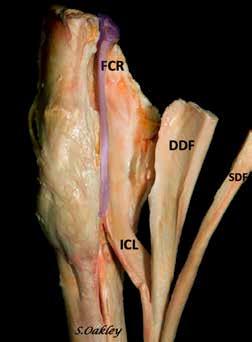

The FCR originates on the medial epicondyle of the humerus and inserts on the second metacarpal bone. The muscle belly is in the antebrachium, and its tendon passes just medial to the carpal sheath. It is rarely injured but is a useful landmark when imaging the area with ultrasound.
The median artery and nerve are within the carpal canal. The median artery is easily visible on ultrasound due to its thick wall. The median vein is superficial to the carpal retinaculum.
Image quality is proportional to patient preparation. The best images will be obtained when the horse is clipped (if the hair is long) and washed with soap before scanning. A high-frequency ultrasound transducer (7 to 15 MHz) at 4-6 cm depth is appropriate for the more superficial structures in most horses, but a convex probe of lower frequency will allow a more complete evaluation of the distal radius and deeper structures.
The SDFT, AL-DDFT, and DDFT should be systematically evaluated in both the long and short axis and the caudal aspect of the radius. It is important to remember the orientation of the ligaments when scanning and align your probe parallel or perpendicular to the long axis of the structure you are examining. (The AL-DDFT runs at an oblique angle to the ground).
The starting point of the examination is often on the medial side of the limb at the level of the chestnut. For ultrasonographers who do not regularly examine this area, it may be easier to orient by starting on the palmar aspect of the limb in the proximal metacarpus and following the SDF proximally.
Begin in the proximal metacarpus and follow the SDF proximally, moving medially around the accessory carpal bone. Continue proximally until the insertion of the ALDDFT is seen separating from the SDFT. To follow the AL-DDFT to its origin, you will have to angle the probe
Figure 1. Soft tissue structures in the carpal canal. FCR – Flexor carpi radialis muscle and tendon, SCL - superior check ligament or accessory ligament of the superficial flexor tendon, SDF - Superficial digital flexor tendon, DDF - Deep digital flexor tendon, FCU - Flexor carpi ulnaris (not in carpal canal). Image courtesy of Dr. Suzan Oakley
perpendicular (or parallel) to the ligament since it is not parallel to the SDF and DDF. There are often linear artifacts in the short axis view from the median artery. The FCR is a good landmark to track your progress. As you progress up the leg, the AL- SDFT will move on the image from its position deep to the median artery to be deep to the FCR just before its origin on the radius. All structures should be examined on the short and long axis. Normal reference images are shown below.

A unique virtual wet lab course featuring a split screen camera with the live probe position and ultrasound image presented simultaneously.
Original anatomical dissections are presented to review and illustrate the relevant anatomy.
Demonstration of HOW to obtain excellent diagnostic images, and practical tips to improve your image quality in the eld.
November 8, 2023 at 7 PM EST
Ultrasound of the Flexor Tendons, Inferior Check Ligament and Fetlock
December 13, 2023 at 7 PM EST
Ultrasound of the Front and Hind Suspensory Ligaments
January 10, 2024 at 7 PM EST
Ultrasound of the Pastern and Foot

February 14, 2024 at 7 PM EST
Ultrasound of the Stie
*RACE approval pending*
For more information and to register, contact Dr. Oakley at equinesportsrehabwellington.com
Figure 3. The arrows depict the approximate level of the ultrasound probe in the reference images below. Level 1 - SDF in the distal carpal canal Level 2 - Insertion of SCL into SDF Level 3 - Mid SCL Level 4 - Near origin of SCL .LEVEL 2
LEVEL 3
LEVEL 4
Legend: SDF - superficial digital flexor tendon, DDF - deep digital flexor tendon, SCL - superior check ligament (AL-SDFT), FCR - flexor carpi radialis, RAD - radius, ACB - accessory carpal bone.










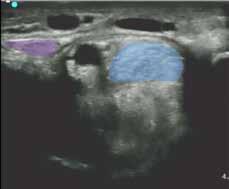
 Figure 4. Images of the carpal canal ultrasound were obtained from simultaneous video of probe position and ultrasound feed. The photos on the left (A) were the same time stamp as the images (B) and (C) for each row. (B) and (C) are the same image; (b) is enhanced with color for clarity and (C) is labeled. The level indicated on each row corresponds to the arrows on the anatomical dissection in Figure 3.
Figure 4. Images of the carpal canal ultrasound were obtained from simultaneous video of probe position and ultrasound feed. The photos on the left (A) were the same time stamp as the images (B) and (C) for each row. (B) and (C) are the same image; (b) is enhanced with color for clarity and (C) is labeled. The level indicated on each row corresponds to the arrows on the anatomical dissection in Figure 3.

• The level of the musculotendinous junction in the SDF can vary slightly from horse to horse. Be careful not to confuse the junction with a lesion in the SDF.

• In a horse with no swelling, it is not always possible to image the origin of the SCL with a tendon probe due to lack of contact with the probe.


• When imaging the superior check ligament (AL-SDFT) in the short axis, be sure to have your probe perpendicular to the ligament, not the ground.
• To examine the caudal radius, it is helpful to flex the limb and use a convex probe.
Figure 5. Ultrasound images of the carpal canal show (A) the vasculature and B) the margins of the carpal retinaculum between the green arrows. M - median artery, C - cephalic vein, dR - distal radial artery and vein. Image courtesy of Dr. Suzan Oakley Figure 6. Long axis view of the superior check ligament (ALSDFT) at 6 cm depth. SCL - superior check ligament, Mamedian artery. Image courtesy of Dr. Suzan Oakley1. Carstens, A. Ultrasonography of the Carpus. In: Kidd, Lu and Frazer, eds. Atlas of Equine Ultrasonography. Sussex, UK: Wiley and Sons, Ltd., 2014; 110-112.
2. Cauvin ERJ, Munroe GA, Boswell J, et al. Gross and ultrasonographic anatomy of the carpal flexor tendon sheath in horses. Vet Rec 1997;141:489–495.

3. Denoix JM, Essentials of the Equine Locomotor System. Boca Raton, FL, USA: CRC Press, 2019;40-47.
4. Denoix JM, Busoni V. Ultrasonographic anatomy of the accessory ligament of the superficial digital flexor tendon in horses. Equine Vet J 1999;31:186–191.
5. Jorgensen JS, Stewart AA, et al. Ultrasonographic examination of the caudal structures of the distal antebrachium in the horse. Equine Vet Educ 2010;22:146–155.

6. Probst A, Macher R, Hinterhofer C, et al. Anatomical features of the carpal flexor retinaculum of the horse. Anat Histol Embryol 2008;37:415–417.
7. Redding WR. Carpal Sheath. In: Stashak TS, ed. Adams and Stashaks’ Lameness in Horses. 6th ed. Sussex, UK: Blackwell Publishing, 2011;545–546.
8. Smith LJ, Mair TS. Rupture of the superficial flexor tendon in the forelimb in aged horses: A report of nine cases. Equine Vet Educ 2007;19:183–186.
9. Vaughan B, Whitcomb MB, Galuppo L, et al. Spontaneous rupture of the proximal superficial digital flexor tendon: A clinical syndrome in aged equids, in Proceedings. Am Assoc Equine Pract 2014;60:257.
10. Wright IM, Minshall GJ. Clinical, radiological and ultrasonographic features, treatment and outcome in 22 horses with caudal distal radial osteochondromata. Equine Vet J 2012;44:319–324.
Suzan Oakley, DVM, Dipl ACVSMR, Dipl ABVP (Equine), Certified Member, ISELP
Dr. Suzan Oakley is a 1991 graduate of the University of Florida. She is the owner of a sports medicine practice in Wellington, Florida. Dr. Oakley is board certified by the American College of Sports Medicine and Rehabilitation and the American Board of Veterinary Practitioners in Equine Practice and is a certified member of the International Society for Equine Locomotor Pathology (ISELP). Dr. Oakley has an avid interest in sport horses, lameness, and the use of ultrasound as a diagnostic tool.
 Figure 8. Short axis view of the DDF at 6 cm depth. SDFsuperficial digital flexor, SCL - superior check ligament (ALSDFT), DDF - deep digital flexor, FCR- flexor carpi radialis, Rad - radius.
Image courtesy of Dr. Suzan Oakley
Figure 8. Short axis view of the DDF at 6 cm depth. SDFsuperficial digital flexor, SCL - superior check ligament (ALSDFT), DDF - deep digital flexor, FCR- flexor carpi radialis, Rad - radius.
Image courtesy of Dr. Suzan Oakley

• The only FDA approved pentosan polysulfate sodium injection
• Convenient; only 4 intramuscular injections required
• Not limited to use for specific joints1

24-hour Veterinary Technical Support available: (866) 933-2472
Nonurgent Technical Support available: support@dechra.com
As with all drugs, side effects may occur. For intramuscular use in horses only. Not for use in humans. Pentosan polysulfate sodium is a weak anticoagulant. Caution should be used when administering Zycosan if you are taking an anticoagulant. In case of accidental self-injection, seek immediate medical attention. If product comes into contact with skin, rinse skin thoroughly with water and seek medical attention if needed. Horses with hypersensitivity to pentosan polysulfate sodium should not receive Zycosan. Do not use Zycosan concurrently with other anticoagulant drugs. Do not use in horses with clotting disorders or within 24 hours of surgical procedures. Caution should be used when administering this drug before or after strenuous activities. Caution should be used when NSAIDS are administered concurrently due to the anticoagulant effects of Zycosan. If Zycosan and NSAIDS are used concurrently, horses should be monitored for hemorrhage or other clinical signs of abnormal bleeding. The safe use of Zycosan has not been evaluated in breeding, pregnant, or lactating horses. The safety of long-term repeat use of Zycosan has not been evaluated. The most frequently reported adverse reactions are injection site reactions, prolongation of coagulation parameters (activated partial thromboplastin time (aPTT) and prothrombin time (PT). Refer to the prescribing information for complete details or visit www.dechra-us.com.

250 mg/mL
For intramuscular use in horses only.
Brief Summary (For Full Prescribing Information, see package insert)
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
DESCRIPTION: Zycosan contains pentosan polysulfate sodium, a semisynthetic polysulfated xylan.
It is a pale yellow to brownish yellow, clear, sterile solution.
INDICATION: For the control of clinical signs associated with osteoarthritis in horses.
CONTRAINDICATIONS: Horses with hypersensitivity to pentosan polysulfate sodium or any of the inactive ingredients in Zycosan should not receive Zycosan. Do not use Zycosan concurrently with other anticoagulant drugs. Do not use in horses with clotting disorders or within 24 hours of surgical procedures (see Warnings and Precautions).
WARNINGS AND PRECAUTIONS:
User Safety Warnings: Not for use in humans. Keep out of reach of children. Pentosan polysulfate sodium is a weak anticoagulant. Caution should be used when administering Zycosan if you are taking an anticoagulant. In case of accidental self-injection, seek immediate medical attention. If product comes into contact with skin, rinse skin thoroughly with water and seek medical attention if needed. To obtain a Safety Data Sheet (SDS), contact Dechra at (866) 933-2472.
Animal Safety Warnings and Precautions:

Zycosan has been shown to prolong coagulation parameters up to 24 hours after injection, therefore caution should be used when administering this drug before or after strenuous activities (see Target Animal Safety). Due to the anticoagulant effects, this drug may exacerbate Exercise Induced Pulmonary Hemorrhage (EIPH).
The concurrent use of NSAID with Zycosan has not been evaluated. Due to the anticoagulant effects of Zycosan and known anticoagulant effects of some NSAIDs, caution should be used if NSAIDs are concurrently administered. Horses concurrently treated with Zycosan and NSAIDs should be monitored for hemorrhage or other clinical signs of abnormal bleeding (e.g., petechiae, ecchymosis, or epistaxis). The safety of long-term repeat use of Zycosan has not been evaluated. Pigmentary changes in the retina (pigmentary maculopathy) have been reported in human patients following long-term oral use of pentosan polysulfate sodium. It is not known if a similar finding occurs in horses. The safe use of Zycosan has not been evaluated in breeding, pregnant, or lactating horses.
Other Warnings: Do not use in horses intended for human consumption.
ADVERSE REACTIONS:
Injection site reactions were the most frequently reported adverse reactions in the field study. Injection site reactions were associated with clinicopathology changes in some cases. Other adverse reactions reported in more than one horse were prolongation of coagulation parameters (activated partial thromboplastin time (aPTT) and prothrombin time (PT)), lethargy, behavior changes, and colic. To report suspected adverse events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Dechra at (866) 933-2472 . For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/reportanimalae.
STORAGE CONDITIONS: Store at room temperature 68-77°F (20-25°C), with excursions to 59-86°F (15-30°C).
MANUFACTURED FOR:
Dechra Veterinary Products
7015 College Boulevard, Suite 525
Overland Park, KS 66211 USA
Approved by FDA under NADA # 141-559
Zycosan is a trademark of Dechra Limited.
R 01 2023
(Hyaluronic Acid/Chondroitin sulfates C4 & C6/ N-acetyl-D-glucosamine Sterile Solution)




OCT. 19-22 | West Palm Beach, FL







You’re invited to join us in West Palm Beach, FL, this October for an equine conference experience like no other.
Promoting Excellence Symposium (PES) is the flagship equine veterinary medical conference of the Florida Association of Equine Practitioners (FAEP) offering equineexclusive, RACE-approved continuing education for the local and global practitioner.

LED BY Jean-Marie
Denoix, DVM, PhD, Assoc. LA-ECVDI, DACVSMR, ISLEP Certified Instructor.
Featuring a hands-on ultrasound wet lab led by the world’s foremost equine musculoskeletal system anatomist, Dr. Jean-Marie Denoix
Presented by nationally and internationally recognized speakers
Maximum of 26 CE hours for veterinarians
Maximum of 23 CE hours for veterinary technicians
Register today to secure your seat and your hotel stay!

This program #20-1080678 is pending AAVSB/RACE approval for 41 continuing education credits. Each 50-minute lecture is equal to one (1) continuing education credit. Conference attendees’ CE hours will be reported to AAVSB/RACEtrack. Florida Board of Veterinary Medicine DBPR Provider #0001682. Sponsor of Continuing Education in New York State.









Dr. Jean-Marie Denoix is the world's leading equine musculoskeletal system anatomist and one of the foremost equine diagnostic ultrasonographers.





Born in 1954 in France, Dr. Denoix grew up helping his father on commercial fishing boats off the coast of Normandy. He studied anatomy and veterinary medicine, graduated from veterinary school in Lyons in 1977, and went on to study anatomy, imaging, and biomechanics. He has lectured extensively on these subjects in over 30 countries. Dr. Denoix is currently Professor of Veterinary Anatomy and Equine Locomotor System Pathology and Rehabilitation at the National Veterinary School in France, an ACVSMR Diplomate and Founding Diplomate of the European College of Veterinary Sport Medicine and Rehabilitation. He pioneered some of equine medicine’s most innovative techniques in imaging, particularly ultrasound, and has published books and more than 40 peer-reviewed publications on these subjects.
Dr. Denoix has been a principal figure in the advancement of equine musculoskeletal diagnostics. Through his teaching and publications, he has enabled practitioners throughout the world to practice an advanced level of diagnostic medicine not previously thought possible.
 DVM,
DVM,




Friday, Oct. 20, 2023 | 7:00 A.M. – 5:00 P.M.
Led by JEAN-MARIE DENOIX
8 CE Credit Hours
INSTRUCTOROur wet lab is your chance to get hands-on ultrasound instruction from imaging experts! Learn to effectively use ultrasound equipment and hone your advanced diagnostic sonography skills.
You will get ample opportunity to practice your skills while supervised by our esteemed experts who will provide helpful tips and feedback.
With six anatomical stations to rotate through, you'll have the chance to practice with ultrasound probes and receive comprehensive instruction in hands-on imaging of various anatomical regions* including:
Foot

Tendon Sheath
Proximal Suspensory Ligaments: Thoracic Limb
Carpal Canal
Hind Suspensory





Stifle
Your registration includes rotation through all stations, breakfast and lunch, and transportation to/from the hotel and the wet lab venue.
With Conference Pass Fee: $995
Wet Lab Only Fee: $1,495
*Subject to change
DVM, PhD, ASSOC. LA-ECVDI, DACVSMR, ISLEP-CERTIFIED
















600 Okeechobee Blvd | West Palm Beach, FL 33401





Hilton West Palm Beach, conveniently located two miles from the Palm Beach waterfront, sets the standard for treating guests and families to endless fun, incredible nearby sights, and an unmatched level of service and attention.



Explore the resort-style pool, enjoy lawn games with the family, ride a bike to Palm Beach, or break a sweat with a group fitness class. Hilton West Palm Beach is all about effortless enjoyment and immediate relaxation, making it the FAEP’s ideal host resort for our incredible conference.
Book your room online by scanning the QR code!



Thank you to our
 Nathan M. Slovis | DVM, DACVIM, CHT Hagyard
Nathan M. Slovis | DVM, DACVIM, CHT Hagyard
At a training facility, you may have animals arriving that are traveling to shows locally as well as nationally. These clients’ animals may have been exposed unknowingly to infectious pathogens. The aim of a good biosecurity program is to prevent the dissemination of these pathogens. In order to prevent the dissemination of disease at your facility, you first must understand the pathology and disease course for the pathogen in question. Second, educate your barn staff about diseases and the importance of hygiene.
As veterinarians, we need to be able to optimize rapid containment for business continuity. Rapid containment will require criteria to identify a sick equid and the trigger points warranting the response for isolation. General recommendations to consider for designation as a response trigger for isolation include:
• Body temperature greater than 101.5ºF (38.6ºC)
• Ataxia or recumbency or other neurologic signs
• Passage of frequent loose feces
• Oral or coronary band vesicular or ulcerative lesions
• Nasal discharge, coughing, and/or lymphadenopathy
• Limb or ventral abdominal wall edema, especially if it occurs in multiple horses
Veterinarians will need to be prepared and have a plan in place should the need for testing become necessary. For example:
• Having the necessary supplies available to promptly implement containment protocols can limit the spread of disease
• Being prepared to perform exams and collect samples
Equine Medical Institute • Communicating with the laboratory regarding protocols for the collection of appropriate samples and shipping prior to testingThe Equine Disease Communication Center’s website (www.equinediseasecc.org) has recommendations for an Isolation Plan including forms on how to do an assessment and developing a detailed plan including supplies for setting up isolation.

Implementation of biosecurity protocols will come at a cost, and decision-makers at all levels will require convincing of its importance. To be successful, it will depend on getting buy-in from all those who have a role in caring for horses and in/on the facility. Education and incentivizing implementation of good biosecurity practices show your commitment to business continuity.
• Remember to lead by example
• Disinfect your hands between horses or use personal protective equipment (PPE) when necessary
• Disinfecting our equipment after use is just one example of how to reduce the risk of an outbreak
Disease spread can vary on the location, pathogen, horses’ housing, and people. This article will go over a variety of causes for dissemination of disease including how equipment can move pathogens if not cleaned between uses.
Disease prevention and control activities for infectious agents occur at four levels:
• Individual
• Institutional
• Community
• Global
The first level known as the individual is predominantly the domain of the primary provider. A variety of prevention strategies can be targeted to individuals through their primary provider. One example is the use of chemoprophylaxis in order to help prevent surgery site infections. The second level is that of the institution which is the domain of the infection-control practitioner or the school health official. This level would include veterinary hospitals, pet boarding facilities, human health care facilities, nursing homes, other human residential facilities, and schools.
Programs to prevent the spread of fecal, respiratory, and blood-borne pathogens to healthcare workers or patients are
examples of control strategies targeted at the institutional level. The third level is targeted to the community (in general) and is predominately the domain of public health agencies (local, state, and national levels). The removal of dead animal carcasses after a hurricane is an example of a control measure targeted at the community. The fourth level is related to global strategies. For a number of important pathogens, it has become evident that global control strategies are critical to have an impact on disease occurrence within the U.S. An example of this is the global strategies for Bovine Spongiform Encephalopathy. Although some control measures are specific for each one of these levels, a substantial overlap can occur. For instance, immunization programs operate at all four levels.
The transmission of infectious agents requires three elements: a source (or reservoir) of the infectious agents, a susceptible host with a portal of entry receptive to the agent, and a mode of transmission for the agent. Identification of areas or processes where transmission of pathogens is likely to occur (control points) and implementation of measures aimed at minimizing the possibility of such transmission, while allowing for reasonable flow and function within the veterinary hospital or animal facility, is an important component of biosecurity.
During the assessment and development of the prevention and control activities targeted to infectious diseases, the weakest link in the chain of infection (agent, transmission, host) needs to be considered for each specific pathogen. In some situations,
control of the agent in a specific reservoir may be the best way to reduce disease occurrence. The chlorination of water is an example of destroying an agent in its reservoir or eliminating a possible mode of transmission. Strategies aimed at the level of transmission need to be tailored to the type of transmission involved. An example of a control activity targeted to airborne transmission is the isolation of the animal to a facility where there is no shared airspace or is located on premises where no other animals are currently housed. The control of vectorborne transmission can be targeted toward destroying the vector and towards the use of repellents. In many instances, the best mechanism to prevent disease occurrence is through modification of the host, such as developing or boosting immunity through active or passive immunization. Other control activities targeted to the host may include improving the nutritional status of a neglected animal or providing chemoprophylaxis against a variety of agents. Every effort should be made to minimize the contact between animals with a history or clinical signs suggestive of infectious contagious disease or those with a confirmed contagious disease and the remainder of the patients or animals at a boarding facility.
Before implementing disease prevention and control strategies, several issues need to be considered which include risk, feasibility, cost, and effectiveness. Risk can be defined by the potential for exposure. Epidemiologic studies or analysis of surveillance data can serve to define animals at risk and can also quantify risk within different populations. Information that is needed for making the optimal decisions regarding
the risk posed would include a history of the arriving animal, history regarding contact of the arriving animal with other animals having a contagious disease, pertinent laboratory testing performed prior to arrival, physical findings on initial examination of the new arrival and pertinent laboratory tests results if available at arrival or soon thereafter. To help better understand the risks of the animal to disseminate a disease there is a need to know the past disease status and exposure status in order to best implement targeted control measures for contagious disease.
Admittance-based alert systems to notify infection control and clinical personnel about readmitted or transferred patients with a history of infection or colonization, and routine assessment of education and training among personnel, can improve infection prevention at the facility level. One such mechanism would be self-certification by the owner or representative of the animal. The owner/authorized caretaker/transporter would sign a form and state the history of the animal in relation to exposure to a specific disorder. The reason for such selfcertification is the inability to establish risk level without this type of information as those receiving the animal would have no mechanism to gather this information other than through self-certification for animals that have no overt signs of disease at the time of presentation to the facility yet the animal could have had exposure to contagious disease agent that would pose a substantial risk to other animals in the facility if not managed accordingly. Risk adjustments require the collection of pertinent information (e.g., salmonella in hospitalized patients, surgery site infections, etc.) about the total population being monitored so that objective data could be used to help direct decisions being made regarding risk assessments. These risk adjustments are important because policies and procedures will need to be updated based on these findings.
In developing control programs, the feasibility of a strategy also needs to be assessed. Feasibility is dependent not only on sociodemographic factors but also on the operating needs of the facility. For instance, there may be equine facilities that buy and sell horses on a routine basis and will accept the risk of contagious disease outbreaks, such as Streptococcus equi, as the option to prevent exposure in newly arrived horses is not an option. Cost and the availability of resources also need to be considered when developing control strategies. Implementing and maintaining even the most basic biosecurity program requires a trained and adequately staffed facility with appropriate supervision. Outbreak investigations have indicated an association between infections and understaffing; the association was consistently linked with poor adherence to hand hygiene. For example, the understaffing of human nurses

can facilitate the spread of MRSA in intensive-care settings through relaxed attention to basic control measures.
Finally, control strategies need to be evaluated for their effectiveness. For example, the effectiveness of the control strategy is a critical issue in evaluating ways to curb salmonellosis in equine care facilities. Cost-effectiveness models are often used in making recommendations for population-based vaccine programs. Challenges to prevention include measurement of outcomes that may be complicated by diagnostic limitations, for example, diagnosis of ventilatorassociated pneumonia in humans.
Prevention strategies for infectious diseases can be characterized by using the traditional concepts of primary, secondary, or tertiary prevention. Primary prevention will be defined as the prevention of infection by personal and community-wide efforts. Secondary prevention includes measures available to individuals and the population for the detection of early infection and effective intervention. Tertiary prevention consists of measures available to reduce or eliminate the long-term impairment and disabilities caused by infectious diseases.
An example of primary prevention is immunoprophylaxis, which can be classified as active or passive.
Active immunoprophylaxis involves the administration of all or part of the microorganism (bacterin) or a product of the microorganism (toxoid) to alter the host by stimulating an immune response aimed at protecting against infection. Active administration is also used in post-exposure situations including immunization after exposure; for example, tetanus. Some of the vaccines may be given in conjunction with an antitoxin in the postexposure setting.
Passive immunization involves the administration of preformed antibodies, often to specific agents, after exposure. The most common forms of passive immunization may be hyperimmunized plasma for a foal with failure of passive transfer or botulism-hyperimmunized plasma.
A second type of primary prevention is antimicrobial prophylaxis often referred to as chemoprophylaxis. The use of effective chemoprophylaxis requires that the infectious agent be susceptible to the antimicrobial used. When used as a primary prevention the medication may be used before or after exposure in order to prevent infection. Examples
of chemoprophylaxis in the post-exposure setting include Rhodococcus equi( e.g. Azithromycin), Clostridium perfringens enterocolitis (e.g. Metronidazole), and Equine Herpes Type 1 Abortion or Neurological (e.g. Valacyclovir). Prophylaxis against surgical wound infections with broad-spectrum antimicrobial coverage before surgery is an example of chemoprophylaxis in a hospital setting.
In many situations, chemoprophylaxis is used because the likelihood of exposure to pathogenic organisms is present, even though proper documentation of exposure is not clear.
Secondary prevention activities entail chemoprophylaxis and involve the identification of early or asymptomatic infection with subsequent treatment so that infections are eradicated, and the sequelae are prevented. Although most secondary prevention programs involve intervention at the individual level through the use of chemoprophylaxis, such programs often operate within the context of populationbased or institutional-based screening efforts. An example of secondary prevention would be the use of procaine penicillin or ceftiofur on horses that test positive for Streptococcus equi on a nasopharyngeal screening wash during a farm outbreak of the disease.
When evaluating hand hygiene products for potential use in health care facilities one must consider factors that can affect the overall efficacy of such products. Studies indicate that the frequency of handwashing or antiseptic washing by personnel

is affected by the accessibility of hand hygiene facilities. It is not uncommon to see some facilities have only one sink available in areas housing many patients or sinks located far away from the patient, which will discourage handwashing by personnel. In contrast, the availability of alcohol-based hand rubs does not require plumbing and can be made available adjacent to patient housing and at many locations in patient care areas.
Failure to perform appropriate hand hygiene is considered the leading cause of healthcare-associated infections and the spread of multi-resistant organisms and has been recognized as a substantial contributor to outbreaks. Of nine hospitalbased studies of the impact of hand hygiene on the risk of healthcare-associated infections, the majority demonstrated a temporal relationship between improved hand hygiene practices and reduced infection rates.
You have diagnosed an infectious agent on the property and now you must decide if isolation and quarantine are necessary. What are the differences between isolation and quarantine? What criteria do you use to determine isolation? Why should you quarantine? How long should you isolate? What horses should be quarantined? Horses may be quarantined if they have had direct or indirect exposure to a sick horse. Indirect exposure would include stabling, personnel, and equipment. The duration of the quarantine depends on the mode of transmission, survivability, and incubation period. Some guidelines for an isolation/quarantine facility.
• The duration of quarantine should, at a minimum, be the incubation period and duration of transmission
• Horses should be moved to an isolation facility if they are sick with a known or presumptive infectious disease


• They would be moved to isolation as soon as they have the first clinical sign
TYPICAL INCUBATION PERIOD AND DURATION OF SHEDDING FOR INFECTIOUS PATHOGENS
EHV1 Typically 4-6 days 7-10 days, up to 21 days
STRANGLES 3-14 days 2-3 weeks (or longer)
INFLUENZA 1-3 days 7-10 days
SAMONELLA 3-5 days Could be weeks to months
CORONAVIRUS 2-4 days 3-25 days (or up to 15 days)
Dr. Nathan Slovis is the Director of the McGee Medical and Critical Care Center at the Hagyard Equine Medical Institute located in Lexington, Kentucky. He is a native of Annapolis, Maryland. He received his Bachelor of Science from Radford University, Doctor of Veterinary Medicine from Purdue University, interned at Arizona Equine Center and completed his residency in Internal Medicine at the University of California, Davis.
Dr. Slovis has published over 60 manuscripts in both national and international peer reviewed veterinary journals. He is the Editor of both the Atlas of Equine Endoscopy and The Atlas of Diseases/Disorders of the Foal both distributed by Elsevier. He implemented the current Infectious Disease and Equine Emergency Response Programs at Hagyard Equine Medical Institute and holds the position of Infectious Disease Officer He is also a Certified Hyperbaric Technologist for humans and animals.
 Nathan M. Slovis DVM, DACVIM, CHT
Nathan M. Slovis DVM, DACVIM, CHT


When disaster strikes, both animals and people are in need of urgent assistance. With the 2023 Atlantic hurricane season in full swing, the Florida Veterinary Corps urgently calls for more veterinarians and technicians to join their cause and support the community during crises.
This volunteer-driven program aids local officials in responding to animal emergencies. Volunteers are an essential lifeline for animals in distress, and their expertise can be the difference between life and death, providing comfort and relief to both animals and their owners when they need it most.

If you are a veterinarian or veterinary technician and able to volunteer, please consider joining the Florida Veterinary Corps before the next natural disaster strikes. By joining the Corps, you have the power to save lives, provide relief, and make a meaningful impact on both animals and people affected by natural disasters.

With vast experience, more resources and greater dedication, Patterson Equine offers unparalleled industry knowledge and support to the customers we serve. Our representatives provide comprehensive insights to help you select the best products, equipment and management tools to run an efficient practice. When it comes to finding the right solutions for your unique needs – we are fully committed to your success.







