REFERENCES
LIVING ON THE EDGE: THE SCIENCE BEHIND THRILL SEEKING BEHAVIORS
1. Zhou, L., Chlebosz, K., Tower, J., & Morris, T. (2020). An exploratory study of motives for participation in extreme sports and physical activity. Journal of Leisure Research, 51(1), 56–76. doi:10.1080/00222216.201 9.1627175.
2. Parry, T., Tolliver, E., & Faucett, S. C. (2019). Extreme Sports. The Sports Medicine Physician, 657–669. doi:10.1007/978-3-030-10433-7_48.
3. Musumeci, G. (2021). Why would you choose to do an extreme sport? Journal of Functional Morphology and Kinesiology, 6(3), 65. doi:10.3390/jfmk6030065.
4. Tofler, I. R., Hyatt, B. M., & Tofler, D. S. (2018). Psychiatric aspects of extreme sports: Three case studies. The Permanente Journal, 22(1), 17–071. doi:10.7812/TPP/17071.
5. Ball, C. M., & Featherstone, P. J. (2017). The early history of adrenaline. Anaesthesia and Intensive Care, 45(3), 279–281. doi:10.1177/0310057X1704500301.
6. Juárez Olguín, H., Calderón Guzmán, D., Hernández García, E., & Barragán Mejía, G. (2016). The role of dopamine and its dysfunction as a consequence of oxidative stress. Oxidative Medicine and Cellular Longevity, 2016, 1–13. doi:10.1155/2016/9730467.
7. Hyman, S. E. (2005). Neurotransmitters. Current Biology, 15(5), R154–R158. doi:10.1016/j.cub.2005.02.037.
8. Verberne, A. J. M., Korim, W. S., Sabetghadam, A., and Llewellyn-Smith, I. J. (2016) Adrenaline: insights into its metabolic roles in hypoglycaemia and diabetes. British Journal of Pharmacology, 173: 1425– 1437. doi:10.1111/bph.13458.
9. Baik, J.-H. (2020). Stress and the dopaminergic reward system. Experimental & Molecular Medicine, 52(12), 1879–1890. doi:10.1038/s12276-020-00532-4.
10. Lewis, R. G., Florio, E., Punzo, D., & Borrelli, E. (2021). The brain’s reward system in health and disease. Advances in Experimental Medicine and Biology, 1344, 57–69. doi:10.1007/978-3-030-81147-1_4.
11. Palmiter, R. D. (2007). Is dopamine a physiologically relevant mediator of feeding behavior? Trends in Neurosciences, 30(8), 375–381.doi:10.1016/j.tins.2007.06.004.
12. Liu, C., Goel, P., & Kaeser, P. S. (2021). Spatial and temporal scales of dopamine transmission. Nature Reviews Neuroscience, 22(6), 345–358. doi:10.1038/ s41583-021-00455-7.
13. Wise, R. A., & Robble, M. A. (2020). Dopamine and
Addiction. Annual Review of Psychology, 71(1), 79–106. doi:10.1146/annurev-psych-010418-103337.
14. Goldstein, D. S. (2010). Adrenal responses to stress. Cellular and Molecular Neurobiology, 30(8), 1433–1440. doi:10.1007/s10571-010-9606-9.
15. Kim, J.-H., & Choi, M. H. (2020). Embryonic development and adult regeneration of the adrenal gland. Endocrinology and Metabolism, 35(4), 765–773. doi:10.3803/ EnM.2020.403.
16. Krahenbuhl, G. S. (1975). Adrenaline, arousal and sport. The Journal of Sports Medicine, 3(3), 117–121. doi:10.1177/ 036354657500300304.
17. Feher, J. (2012). The adrenal medulla and integration of metabolic control. Quantitative Human Physiology, 916–923. doi:10.1016/B978-0-12-800883-6.00089-6.
18. Vadas, D., Kalichman, L., Hadanny, A., & Efrati, S. (2017). Hyperbaric oxygen environment can enhance brain activity and multitasking performance. Frontiers in Integrative Neuroscience, 11, 25. doi:10.3389/fnint.2017.00025.
19. Buckley, R. (2012). Rush as a key motivation in skilled adventure tourism: Resolving the risk recreation paradox. Tourism Management, 33(4), 961–970. doi:10.1016/j. tourman.2011.10.002.
20. Robbins, T., & Clark, L. (2015). Behavioral addictions. Current Opinion in Neurobiology, 30, 66–72. doi:10.1016/j. conb.2014.09.005.
21. Leeman, R. F., & Potenza, M. N. (2013). A targeted review of the neurobiology and genetics of behavioural addictions: An emerging area of research. The Canadian Journal of Psychiatry, 58(5), 260–273. doi:10.1177/070674371 305800503.
22. Gardner, M., & Steinberg, L. (2005). Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: An experimental study. Developmental Psychology, 41(4), 625–635. doi:10.1037/0012-1649.41.4.625
23. Tian, L., Guo, M., Lu, Y., Liu, L., & Lu, Y. (2022). Risk-taking behavior among male adolescents: The role of observer presence and individual self-control. Journal of Youth and Adolescence, 51(11), 2161–2172. doi:10.1007/s10964022-01659-5.
24. Salas-Rodríguez, J., Gómez-Jacinto, L., Hombrados-Mendieta, I., & Del Pino-Brunet, N. (2022). Applying an evolutionary approach of risk-taking behaviors in adolescents. Frontiers in Psychology, 12, 694134. doi:10.3389/fpsyg.2021.694134.
25. Tøstesen, G., & Langseth, T. (2021). Freeride skiing— Risk-taking, recognition, and moral boundaries. Frontiers in Sports and Active Living, 3, 650564. doi:10.3389/ fspor.2021.650564.
26. Brymer, E. (2010). Risk taking in extreme sports: A phenomenological perspective. Annals of Leisure Research, 13(1–2), 218–238. doi:10.1080/11745398.2010.9686845.
GREY MATTERS JOURNAL AT VASSAR COLLEGE | ISSUE 6 48 REFERENCES
LOVE ON THE BRAIN: THE SCIENCE OF LUST, ATTRACTION, AND ATTACHMENT
1. Fisher, E. E., Aron, A., Mashek, D., Haifang, L., & Brown, L. L. (2002). Defining the brain systems of lust, romantic attraction, and attachment. Archives of Sexual Behavior, 31(5), 413-419. doi:10.1023/A:1019888024255.
2. Georgiadis, J. R. (2012). Doing it … wild? on the role of the cerebral cortex in human sexual activity. Socioaffective Neuroscience & Psychology, 2(1), 17337. doi:10.3402/snp.v2i0.17337.
3. Calabrò, R. S., Cacciola, A., Bruschetta, D., Milardi, D., Quattrini, F., Sciarrone, F., Rosa, G., Bramanti, P., & Anastasi, G. (2019). Neuroanatomy and function of human sexual behavior: A neglected or unknown issue? Brain and Behavior, 9(12). doi:10.1002/brb3.1389.
4. Saper, C. B., & Lowell, B. B. (2014). The hypothalamus. Current Biology, 24(23), 1111-1116. doi:10.1016/j. cub.2014.10.023.
5. Graziottin, A. (2000). Libido: The biologic scenario. Maturitas, 34. doi:10.1016/s0378-5122(99)00072-9.
6. Seshadri, K. G. (2016). The neuroendocrinology of Love. Indian Journal of Endocrinology and Metabolism, 20(4), 558. doi:10.4103/2230-8210.183479.
7. Forbes, C. E., & Grafman, J. (2010). The role of the human prefrontal cortex in social cognition and moral judgment. Annual Review of Neuroscience, 33(1), 299–324. doi:10.1146/annurev-neuro-060909-153230.
8. Fisher, H., Aron, A., & Brown, L. L. (2005). Romantic love: An fmri study of a neural mechanism for mate choice. The Journal of Comparative Neurology, 493(1), 58–62. doi:10.1002/cne.20772.
9. Finke, J. B., Behrje, A., & Schächinger, H. (2018). Acute stress enhances pupillary responses to erotic nudes: Evidence for differential effects of sympathetic activation and cortisol. Biological Psychology, 137, 73–82. doi:10.1016/j.biopsycho.2018.07.005.
10. Jouffre, S. (2014). Power modulates over-reliance on false cardiac arousal when judging target attractiveness. Personality and Social Psychology Bulletin, 41(1), 116–126. doi:10.1177/0146167214559718.
11. AlAwlaqi, A., Amor, H., & Hammadeh, M. E. (2017). Role of hormones in hypoactive sexual desire disorder and current treatment. Journal of the Turkish-German Gynecological Association, 18(4), 210–218. doi:10.4274/ jtgga.2017.0071.
12. Chichinadze, K., & Chichinadze, N. (2008). Stress-induced increase of testosterone: Contributions of social status and sympathetic reactivity. Physiology & Behavior, 94(4), 595–603. doi:10.1016/j.physbeh.2008.03.020.
13. Marazziti, D., & Canale, D. (2004). Hormonal changes when falling in Love. Psychoneuroendocrinology,
29(7), 931–936. doi:/10.1016/j.psyneuen.2003.08.006.
14. Péloquin, K., Brassard, A., Delisle, G., & Bédard, M. (2013). Integrating the attachment, caregiving, and sexual systems into the understanding of sexual satisfaction. Canadian Journal of Behavioral Science, 45(3), 185-195. doi:10.1037/a0033514.
15. Diamond, L. M. (2003). What does sexual orientation orient? A biobehavioral model distinguishing romantic love and sexual desire. Psychological Review, 110(1), 173-192. doi:10.1037/0033-295X.110.1.173.
16. Emerging perspectives on distinctions between romantic love and sexual desire. Current Directions in Psychological Science, 13(3), 116–119. doi:10.1111/j.09637214.2004.00287.x.
17. Acevedo, B. P., Poulin, M. J., Collins, N. L. & Brown, L. L. (2020). After the honeymoon: neural and genetic correlates of romantic love in newlywed marriages. Frontiers in Psychology, 11. doi:10.3389/fpsyg.2020.00634.
18. Gander, M., & Buchheim, A. (2015). Attachment classification, psychophysiology and frontal EEG asymmetry across the lifespan: A Review. Frontiers in Human Neuroscience, 9. doi:10.3389/fnhum.2015.00079.
19. Botvinick, M. M., Cohen, J. D., & Carter, C. S. (2004). Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences, 8(12), 539–546. doi:10.1016/j.tics.2004.10.003.
20. Maddock, R. J. (1999). The retrosplenial cortex and emotion: New insights from functional neuroimaging of the human brain. Trends in Neurosciences, 22(7), 310–316. doi:10.1016/s0166-2236(98)01374-5.
21. Winston J. S., Strange B. A., O’Doherty J., Dolan R. J. (2002). Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat. Neurosci. 5, 277–283. doi:10.1038/nn816.
22. Aron, A., Fisher, H., Mashek, D. J., Strong, G., Li, H., & Brown, L. L. (2005). Reward, motivation, and emotion systems associated with early-stage intense romantic love. Journal of Neurophysiology, 94(1), 327–337. doi:10.1152/jn.00838.2004.
23. Glover J. A., Galliher, R. V., & Crowell, K. A. (2015). Young women’s passionate friendships: a qualitative analysis. Journal of Gender Studies, 24(1), 70-84. doi:10.1080/095 89236.2013.820131.
24. Suleiman, A. B., Galván, A., Harden, K. P., & Dahl, R. E. (2017). Becoming a sexual being: the ‘elephant in the room’ of adolescent brain development. Development of Cognitive Neuroscience, 25, 209-220. doi:10.1016/j. dcn.2016.09.004.
25. Love, T. M. (2013). Oxytocin, motivation, and the role of dopamine. Pharmacology Biochemistry and Behavior, 0, 49-60. doi:10.1016/j.pbb.2013.06.011.
26. 25. Czernecka, J. (2021). The social definition of love and its role in maintaining and intimate relationship: Typology of attitudes towards love. Acta Universitatis Lodzien-
GREY MATTERS JOURNAL AT VASSAR COLLEGE | ISSUE 6 49 REFERENCES
REFERENCES
sis. Folia Sociologica, (79), 121–132. doi:10.18778/0208600x.79.07.
27. 26. Moors, A. C., Gesselman, A. N., & Garcia, J. R. (2021). Desire, familiarity, and engagement in polyamory: Results from a national sample of single adults in the United States. Frontiers in Psychology, 12. doi:10.3389/ fpsyg.2021.619640.
THE BRAIN-COMPUTER DEBATE: IS THE HUMAN BRAIN LIKE A COMPUTER?
1. Oppy, G., & Dowe, D. (2021). The Turing Test. Stanford Encyclopedia of Philosophy.
2. Brette, R. (2022). Brains as computers: metaphor, analogy, theory or fact? Frontiers in Ecology and Evolution, 10. doi:10.3389/fevo.2022.878729
3. Falk, D. (2021). Is your brain a computer? MIT Technology Review.
4. Cragon, H. G. (2000). Computer Overview. Computer Architecture and Implementation (pp. 1–17). Cambridge University Press.
5. Pastur-Romay, L. A., Porto-Pazos, A. B., Cedron, F., & Pazos, A. (2017). Parallel Computing for Brain Simulation. Current Topics in Medicinal Chemistry, 17(14), 1646–1668. doi:10.2174/1568026617666161104105725
6. Chen, D., Hu, Y., Cai, C., Zeng, K., & Li, X. (2016). Brain Big Data Processing with Massively Parallel Computing Technology: Challenges and opportunities. Software: Practice and Experience, 47(3), 405–420. doi:10.1002/ spe.2418
7. Winston, P. H. (2016). Marvin L. Minsky (1927–2016). Nature, 530(7590), 282–282. doi:10.1038/530282a
8. Minsky, M. L. (2007). The Society of Mind. Simon & Schuster Paperbacks.
9. Davis, J. I., & Markman, A. B. (2012). Embodied cognition as a practical paradigm: introduction to the topic, the future of embodied cognition. Topics in Cognitive Science, 4(4), 685–691. doi:10.1111/j.17568765.2012.01227.x
10. Hawkins, J., & Blakeslee, S. (2004). On Intelligence. Owl Books.
11. Chen, Y., & Swanson, R. A. (2003). Astrocytes and brain injury. Journal of Cerebral Blood Flow & Metabolism, 137–149. doi:10.1097/00004647-200302000-00001
12. Filosa, A., Paixão, S., Honsek, S. D., Carmona, M. A., Becker, L., Feddersen, B., Gaitanos, L., Rudhard, Y., Schoepfer, R., Klopstock, T., Kullander, K., Rose, C. R., Pasquale, E. B., & Klein, R. (2009). Neuron-glia communication via EphA4/ephrin-A3 modulates LTP through glial glutamate transport. Nature Neuroscience, 12(10), 1285–1292. doi:10.1038/nn.2394
13. Halassa, M. M., & Haydon, P. G. (2010). Integrated brain circuits: astrocytic networks modulate neuronal activ-
ity and behavior. Annual Review of Physiology, 72, 335–355. doi:10.1146/annurev-physiol-021909-135843
14. Dulamea A. O. (2017). Role of Oligodendrocyte Dysfunction in Demyelination, Remyelination and Neurodegeneration in Multiple Sclerosis. Advances in Experimental Medicine and Biology, 958, 91–127. doi: 10.1007/978-3319-47861-6_7
15. Alonso, A. D., Cohen, L. S., Corbo, C., Morozova, V., El-Idrissi, A., Phillips, G., & Kleiman, F. E. (2018). Hyperphosphorylation of tau associates with changes in its function beyond microtubule stability. Frontiers in Cellular Neuroscience, 12. doi:10.3389/fncel.2018.00338
16. Naseri, N. N., Wang, H., Guo, J., Sharma, M., & Luo, W. (2019). The complexity of tau in Alzheimer’s disease. Neuroscience Letters, 705, 183–194. doi:10.1016/j. neulet.2019.04.022
17. Breijyeh, Z., & Karaman, R. (2020). Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules, 25(24), 5789. doi:10.3390/molecules25245789
18. Dzamba, D., Harantova, L., Butenko, O., & Anderova, M. (2016). Glial Cells - The Key Elements of Alzheimer’s Disease. Current Alzheimer Research, 13(8), 894–911. doi:10. 2174/1567205013666160129095924
19. Yampolskiy, R. (2020, December 14). Can computers evolve to program themselves without programmers? Mind Matters.
20. Zhong, X., Shi, H., Hou, L., Chen, B., Peng, Q., Chen, X., Wu, Z., Wang, Y., Mai, N., Huang, X., & Ning, Y. (2017). Neuropsychiatric Features of Neurosyphilis: Frequency, Relationship with the Severity of Cognitive Impairment and Comparison with Alzheimer Disease. Dementia and geriatric cognitive disorders, 43(5-6), 308–319. doi: 10.1159/000476060
21. Bronas, U. G., Puzantian, H., & Hannan, M. (2017). Cognitive Impairment in Chronic Kidney Disease: Vascular Milieu and the Potential Therapeutic Role of Exercise. BioMed Research International, 2017, 2726369. doi:10.1155/2017/2726369
22. Joshee, P., Wood, A. G., Wood, E. R., & Grunfeld, E. A. (2018). Meta-analysis of cognitive functioning in patients following kidney transplantation. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association, 33(7), 1268–1277. doi: 10.1093/ndt/gfx240
23. Luo, M., Zeng, Q., Jiang, K., Zhao, Y., Long, Z., Du, Y., Wang, K., & He, G. (2021). Estrogen deficiency exacerbates learning and memory deficits associated with glucose metabolism disorder in APP/PS1 double transgenic female mice. Genes & Diseases, 9(5), 1315–1331. doi:10.1016/j.gendis.2021.01.007
24. Brueckner, A. L. (1986). Brains in a Vat. The Journal of Philosophy, 83(3), 148–167. doi:10.2307/2026572
25. Putnam, H. (2009). Brains in a Vat. In S. Bernecker & F. I. Dretske (Eds.), Knowledge: Readings in contemporary
GREY MATTERS JOURNAL AT VASSAR COLLEGE | ISSUE 6 50
epistemology. essay, Oxford University Press.
26. Stewart, J. R., Gapenne, O., & Di Paolo, E. (2014). Enaction: Toward a new paradigm for cognitive science. MIT Press.
GRIEVING A CHANGING ENVIRONMENT: EXPLORING THE EFFECTS OF CLIMATE CHANGE ON THE PSYCHE
1. Warsini, S., Mills, J., & Usher, K. (2014). Solastalgia: Living With the Environmental Damage Caused By Natural Disasters. Prehospital and Disaster Medicine, 29(1), 87-90. doi:10.1017/S1049023X13009266.
2. Albrecht, G., Sartore, G.-M., Connor, L., Higginbotham, N., Freeman, S., Kelly, B., Stain, H., Tonna, A., & Pollard, G. (2007). Solastalgia: The distress caused by environmental change. Australasian Psychiatry, 15(1_suppl). doi:10.1080/10398560701701288.
3. Comtesse, H., Ertl, V., Hengst, S. M., Rosner, R., & Smid, G. E. (2021). Ecological grief as a response to environmental change: A mental health risk or functional response? International Journal of Environmental Research and Public Health, 18(2), 734. doi:10.3390/ ijerph18020734.
4. Leal Filho, W., Matandirotya, N. R., Lütz, J. M., Alemu, E. A., Brearley, F. Q., Baidoo, A. A., Kateka, A., Ogendi, G. M., Adane, G. B., Emiru, N., & Mbih, R. A. (2021). Impacts of climate change to African indigenous communities and examples of adaptation responses. Nature Communications, 12(1), 6224. doi:10.1038/ s41467-021-26540-0.
5. Obradovich, N., Migliorini, R., Paulus, M. P., & Rahwan, I. (2018). Empirical evidence of mental health risks posed by climate change. Proceedings of the National Academy of Sciences, 115(43), 10953–10958. doi:10.1073/pnas.1801528115.
6. Galway, L. P., Beery, T., Jones-Casey, K., & Tasala, K. (2019). Mapping the Solastalgia Literature: A Scoping Review Study. International Journal of Environmental Research and Public Health, 16(15), 2662. doi:10.3390/ ijerph16152662.
7. Bonanno, G. A., & Kaltman, S. (2001). The varieties of grief experience. Clinical Psychology Review, 21(5), 705–734. doi:10.1016/s0272-7358(00)00062-3.
8. Peña-Vargas, C., Armaiz-Peña, G., & Castro-Figueroa, E. (2021). A biopsychosocial approach to grief, depression, and the role of emotional regulation. Behavioral Sciences, 11(8), 110. doi:10.3390/bs11080110.
9. Ágoston, C., Csaba, B., Nagy, B., Kőváry, Z., Dúll, A., Rácz, J., & Demetrovics, Z. (2022). Identifying Types of Eco-Anxiety, Eco-Guilt, Eco-Grief, and Eco-Coping in a Climate-Sensitive Population: A Qualitative Study. International Journal of Environmental Research and Public Health, 19(4), 2461. doi:10.3390/ijerph19042461.
10. Clayton, S. (2020). Climate anxiety: Psychological re-
sponses to climate change. Journal of Anxiety Disorders, 74, 102263. doi:10.1016/j.janxdis.2020.102263
11. Cianconi, P., Betrò, S., & Janiri, L. (2020). The impact of climate change on Mental Health: A Systematic Descriptive Review. Frontiers in Psychiatry, 11. doi:10.3389/fpsyt.2020.00074.
12. Genkinger, J. M., Stigter, L., Jedrychowski, W., Huang, T.-J., Wang, S., Roen, E. L., Majewska, R., Kieltyka, A., Mroz, E., & Perera, F. P. (2015). Prenatal polycyclic aromatic hydrocarbon (PAH) exposure, antioxidant levels and behavioral development of children ages 6–9. Environmental Research, 140, 136–144. doi:10.1016/j.envres.2015.03.017.
13. Schwaab, L., Gebhardt, N., Friederich, H.-C., & Nikendei, C. (2022). Climate change related depression, anxiety and stress symptoms perceived by medical students. International Journal of Environmental Research and Public Health, 19(15), 9142. doi:10.3390/ijerph19159142.
14. Shear, K., & Shair, H. (2005). Attachment, loss, and complicated grief. Developmental Psychobiology, 47(3), 253–267. doi:10.1002/dev.20091.
15. Iqbal, T., Elahi, A., Wijns, W., & Shahzad, A. (2023). Cortisol detection methods for stress monitoring in Connected Health. Health Sciences Review, 6, 100079. doi:10.1016/j. hsr.2023.100079.
16. Segerstrom, S. C., & Miller, G. E. (2004). Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin, 130(4), 601–630. doi:10.1037/0033-2909.130.4.601.
17. O’Connor M. F. (2019). Grief: A Brief History of Research on How Body, Mind, and Brain Adapt. Psychosomatic Medicine, 81(8), 731–738. doi:10.1097/PSY.0000000000000717.
18. Saavedra Pérez, H. C., Ikram, M. A., Direk, N., Prigerson, H. G., Freak-Poli, R., Verhaaren, B. F., Hofman, A., Vernooij, M., & Tiemeier, H. (2015). Cognition, structural brain changes and complicated grief. A population-based study. Psychological Medicine, 45(7), 1389–1399. doi:10.1017/S0033291714002499.
19. Schlegel, L. M. (2022). Between climates of fear and blind optimism: The affective role of emotions for climate (in)action. Geographica Helvetica, 77(4), 421–431. doi:10.5194/gh-77-421-2022.
20. Dodds, J. (2021). The psychology of climate anxiety. BJPsych Bulletin, 45(4), 222-226. doi:10.1192/bjb.2021.18
21. Foote, K. E., & Azaryahu, M. (2009). Sense of place. International Encyclopedia of Human Geography, 96–100. doi:10.1016/b978-008044910-4.00998-6.
22. Ratdomopurbo, A., Beauducel, F., Subandriyo, J., Agung Nandaka, I. G. M., Newhall, C. G., Suharna, Sayudi, D. S., Suparwaka, H., & Sunarta. (2013). Overview of the 2006 eruption of Mt. Merapi. Journal of Volcanology and Geothermal Research, 261, 87–97. doi:10.1016/j.jvolgeores.2013.03.019.
23. Lawrance, E. L., Jennings, N., Kioupi, V., Thompson, R.,
GREY MATTERS JOURNAL AT VASSAR COLLEGE | ISSUE 6 51 REFERENCES
REFERENCES
Diffey, J., & Vercammen, A. (2022). Psychological responses, mental health, and sense of agency for the dual challenges of climate change and the COVID-19 pandemic in young people in the UK: an online survey study. The Lancet. Planetary health, 6(9), e726–e738. doi:10.1016/S2542-5196(22)00172-3.
24. Mei, E. T., Fajarwati, A., Hasanati, S., & Sari, I. M. (2016). Resettlement following the 2010 Merapi Volcano Eruption. Procedia - Social and Behavioral Sciences, 227, 361–369. doi:10.1016/j.sbspro.2016.06.083.
25. Warsini, S., Buettner, P., Mills, J., West, C., & Usher, K. (2014). The psychosocial impact of the environmental damage caused by the Mt Merapi eruption on survivors in Indonesia. EcoHealth, 11(4), 491–501. doi:10.1007/ s10393-014-0937-8.
26. McNamara, K. E., & Westoby, R. (2011). Solastalgia and the gendered nature of climate change: An example from Erub Island, Torres Strait. EcoHealth, 8(2), 233–236. doi:10.1007/s10393-011-0698-6
HYPOXIC CONDITIONING: WHEN LESS (OXYGEN) IS MORE
1. Thielke, S., Slatore, C. G., & Banks, W. A. (2015). Association between Alzheimer dementia mortality rate and altitude in California counties. JAMA Psychiatry, 72(12), 1253–1254. doi:10.1001/jamapsychiatry.2015.1852.
2. Haque, R. U., & Levey, A. I. (2019). Alzheimer’s disease: a clinical perspective and future nonhuman primate research opportunities. Proceedings of the National Academy of Sciences, 116(52), 26224–26229. doi:10.1073/pnas.1912954116.
3. Mallet, R. T., Manukhina, E. B., Ruelas, S. S., Caffrey, J. L., & Downey, H. F. (2018). Cardioprotection by intermittent hypoxia conditioning: evidence, mechanisms, and therapeutic potential. American Journal of Physiology-Heart and Circulatory Physiology, 315(2), H216–H232. doi:10.1152/ajpheart.00060.2018.
4. Rhudy, J. P., Bakitas, M. A., Hyrkäs, K., Jablonski‐Jaudon, R. A., Pryor, E. R., Wang, H. E., & Alexandrov, A. W. (2015). Effectiveness of regionalized systems for stroke and myocardial infarction. Brain and Behavior, 5(10), e00398. doi:10.1002/brb3.398.
5. Mitroshina, E. V., Savyuk, M. O., Ponimaskin, E., & Vedunova, M. V. (2021). Hypoxia-inducible factor (HIF) in ischemic stroke and neurodegenerative disease. Frontiers in Cell and Developmental Biology, 9, 703084. doi:10.3389/fcell.2021.703084.
6. Weller, J., & Budson, A. (2018). Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research, 7, 1161. doi:10.12688/f1000research.14506.1.
7. Yiannopoulou, K. G., & Papageorgiou, S. G. (2020). Current and future treatments in Alzheimer disease: an update. Journal of Central Nervous System Disease,
12. doi:10.1177/1179573520907397.
8. Magistretti, P. J., & Allaman, I. (2013). Brain energy metabolism. In D. W. Pfaff (Ed.), Neuroscience in the 21st Century: From Basic to Clinical, 1591-1620. doi:10.1007/9781-4614-1997-6_56.
9. Watts, M. E., Pocock, R., & Claudianos, C. (2018). Brain energy and oxygen metabolism: emerging role in normal function and disease. Frontiers in Molecular Neuroscience, 11, 216. doi:10.3389/fnmol.2018.00216.
10. Neuhaus, C., & Hinkelbein, J. (2014). Cognitive responses to hypobaric hypoxia: implications for aviation training. Psychology Research and Behavior Management, 7, 297–302. doi:10.2147/PRBM.S51844.
11. Woodrow, A. D., Webb, J. T., & Wier, G. S. (2011). Recollection of hypoxia symptoms between training events. Aviation, Space, and Environmental Medicine, 82(12), 1143–1147. doi:10.3357/asem.2987.2011.
12. Verges, S., Chacaroun, S., Godin-Ribuot, D., & Baillieul, S. (2015). Hypoxic conditioning as a new therapeutic modality. Frontiers in Pediatrics, 3. doi:10.3389/ fped.2015.00058.
13. Rybnikova, E., & Samoilov, M. (2015). Current insights into the molecular mechanisms of hypoxic pre- and postconditioning using hypobaric hypoxia. Frontiers in Neuroscience, 9, 388. doi:10.3389/fnins.2015.00388.
14. Smith, Z. M., Krizay, E., Guo, J., Shin, D. D., Scadeng, M., & Dubowitz, D. J. (2013). Sustained high-altitude hypoxia increases cerebral oxygen metabolism. Journal of Applied Physiology, 114(1), 11–18. doi:10.1152/japplphysiol.00703.2012.
15. Vestergaard, M. B., Lindberg, U., Aachmann-Andersen, N. J., Lisbjerg, K., Christensen, S. J., Law, I., Rasmussen, P., Olsen, N. V., & Larsson, H. B. (2016). Acute hypoxia increases the cerebral metabolic rate – a magnetic resonance imaging study. Journal of Cerebral Blood Flow & Metabolism, 36(6), 1046–1058. doi:10.1177/0271678X15606460.
16. Xu, F., Liu, P., Pascual, J. M., Xiao, G., & Lu, H. (2012). Effect of hypoxia and hyperoxia on cerebral blood flow, blood oxygenation, and oxidative metabolism. Journal of Cerebral Blood Flow & Metabolism, 32(10), 1909–1918. doi:10.1038/jcbfm.2012.93.
17. Merelli, A., Rodríguez, J. C. G., Folch, J., Regueiro, M. R., Camins, A., & Lazarowski, A. (2018). Understanding the role of hypoxia inducible factor during neuro-degeneration for new therapeutics opportunities. Current Neuropharmacology, 16(10), 1484–1498. doi:10.2174/157015 9X16666180110130253.
18. Jain, I. H., Zazzeron, L., Goli, R., Alexa, K., SchatzmanBone, S., Dhillon, H., Goldberger, O., Peng, J., Shalem, O., Sanjana, N. E., Zhang, F., Goessling, W., Zapol, W. M., & Mootha, V. K. (2016). Hypoxia as a therapy for mitochondrial disease. Science, 352(6281), 54–61. doi:10.1126/science.aad9642.
19. Liu, M., & Alkayed, N. J. (2005). Hypoxic precondition-
GREY MATTERS JOURNAL AT VASSAR COLLEGE | ISSUE 6 52
ing and tolerance via hypoxia inducible factor (HIF) 1 -linked induction of P450 2C11 epoxygenase in astrocytes. Journal of Cerebral Blood Flow & Metabolism, 25(8), 939–948. doi:10.1038/sj.jcbfm.9600085.
20. Millán, I., Corral-Debrisky, M., Vento, M., & Torres-Cuevas, I. (2022). Hypoxic preconditioning induces neuroprotection against oxidative stress. Redox Experimental Medicine, 2022(1), R159–R167. doi:10.1530/ REM-22-0011.
21. Jung, M. E., Simpkins, J. W., Wilson, A. M., Downey, H. F., & Mallet, R. T. (2008). Intermittent hypoxia conditioning prevents behavioral deficit and brain oxidative stress in ethanol-withdrawn rats. Journal of Applied Physiology, 105(2), 510–517. doi:10.1152/japplphysiol.90317.2008.
22. Manukhina, E. B., Goryacheva, A. V., Barskov, I. V., Viktorov, I. V., Guseva, A. A., Pshennikova, M. G., Khomenko, I. P., Mashina, S. Yu., Pokidyshev, D. A., & Malyshev, I. Yu. (2010). Prevention of neurodegenerative damage to the brain in rats in experimental Alzheimer’s disease by adaptation to hypoxia. Neuroscience and Behavioral Physiology, 40(7), 737–743. doi:10.1007/s11055-0109320-6.
23. Shu, L., Wang, C., Wang, J., Zhang, Y., Zhang, X., Yang, Y., Zhuo, J., & Liu, J. (2016). The neuroprotection of hypoxic preconditioning on rat brain against traumatic brain injury by up-regulated transcription factor Nrf2 and HO-1 expression. Neuroscience Letters, 611, 74–80. doi:10.1016/j.neulet.2015.11.012.
24. Biswal, S., Sharma, D., Kumar, K., Nag, T. C., Barhwal, K., Hota, S. K., & Kumar, B. (2016). Global hypoxia induced impairment in learning and spatial memory is associated with precocious hippocampal aging. Neurobiology of Learning and Memory, 133, 157–170. doi:10.1016/j.nlm.2016.05.011.
25. Stowe, A. M., Altay, T., Freie, A. B., & Gidday, J. M. (2011). Repetitive hypoxia extends endogenous neurovascular protection for stroke. Annals of Neurology, 69(6), 975–985. doi:10.1002/ana.22367.
26. Brookmeyer, R., Johnson, E., Ziegler-Graham, K., & Arrighi, H. M. (2007). Forecasting the global burden of Alzheimer’s disease. Alzheimer’s & Dementia, 3(3), 186–191. doi:10.1016/j.jAlz.2007.04.381.
27. Camandola, S., & Mattson, M. P. (2017). Brain metabolism in health, aging, and neurodegeneration. The EMBO Journal, 36(11), 1474–1492. doi:10.15252/ embj.201695810.
28. Denver, P., & McClean, P. L. (2018). Distinguishing normal brain aging from the development of Alzheimer’s disease: inflammation, insulin signaling and cognition. Neural Regeneration Research, 13(10), 1719–1730. doi:10.4103/1673-5374.238608.
29. Leparulo, A., Bisio, M., Redolfi, N., Pozzan, T., Vassanelli, S., & Fasolato, C. (2022). Accelerated aging char-
acterizes the early stage of Alzheimer’s disease. Cells, 11(2), 238. doi:10.3390/cells11020238.
30. Korte, N., Nortley, R., & Attwell, D. (2020). Cerebral blood flow decrease as an early pathological mechanism in Alzheimer’s disease. Acta Neuropathologica, 140(6), 793–810. doi:10.1007/s00401-020-02215-w.
31. Cardoso, S., Carvalho, C., Correia, S. C., Seiça, R. M., & Moreira, P. I. (2016). Alzheimer’s disease: from mitochondrial perturbations to mitochondrial medicine. Brain Pathology, 26(5), 632–647. doi:10.1111/bpa.12402.
32. Tang, J., Oliveros, A., & Jang, M.-H. (2019). Dysfunctional mitochondrial bioenergetics and synaptic degeneration in Alzheimer disease. International Neurourology Journal, 23(Suppl 1), S5-S10. doi:10.5213/inj.1938036.018.
33. Rybnikova, E., Vataeva, L., Tyulkova, E., Gluschenko, T., Otellin, V., Pelto-Huikko, M., & Samoilov, M. O. (2005). Mild hypoxia preconditioning prevents impairment of passive avoidance learning and suppression of brain NGFI-A expression induced by severe hypoxia. Behavioural Brain Research, 160(1), 107–114. doi:10.1016/j. bbr.2004.11.023.
34. Rybnikova, E. A., Samoilov, M. O., Mironova, V. I., Tyul’kova, E. I., Pivina, S. G., Vataeva, L. A., Ordyan, N. É., Abritalin, E. Yu., & Kolchev, A. I. (2008). The possible use of hypoxic preconditioning for the prophylaxis of poststress depressive episodes. Neuroscience and Behavioral Physiology, 38(7), 721–726. doi:10.1007/s11055-0089038-x.
35. Manukhina, E. B., Downey, H. F., Shi, X., & Mallet, R. T. (2016). Intermittent hypoxia training protects cerebrovascular function in Alzheimer’s disease. Experimental Biology and Medicine, 241(12), 1351–1363. doi:10.1177/1535370216649060.
36. Correia, S. C., Machado, N. J., Alves, M. G., Oliveira, P. F., & Moreira, P. I. (2021). Intermittent hypoxic conditioning rescues cognition and mitochondrial bioenergetic profile in the triple transgenic mouse model of Alzheimer’s disease. International Journal of Molecular Sciences, 22(1), 461. doi:10.3390/ijms22010461.
37. Roher, A. E., Debbins, J. P., Malek-Ahmadi, M., Chen, K., Pipe, J. G., Maze, S., Belden, C., Maarouf, C. L., Thiyyagura, P., Mo, H., Hunter, J. M., Kokjohn, T. A., Walker, D. G., Kruchowsky, J. C., Belohlavek, M., Sabbagh, M. N., & Beach, T. G. (2012). Cerebral blood flow in Alzheimer’s disease. Vascular Health and Risk Management, 8, 599–611. doi:10.2147/VHRM.S34874.
38. Liguori, C., Chiaravalloti, A., Sancesario, G., Stefani, A., Sancesario, G. M., Mercuri, N. B., Schillaci, O., & Pierantozzi, M. (2016). Cerebrospinal fluid lactate levels and brain [18F]FDG PET hypometabolism within the default mode network in Alzheimer’s disease. European Journal of Nuclear Medicine and Molecular Imaging, 43(11), 2040–2049. doi:10.1007/s00259-016-3417-2.
39. Yuan, G., Nanduri, J., Khan, S., Semenza, G. L., & Pra-
GREY MATTERS JOURNAL AT VASSAR COLLEGE | ISSUE 6 53 REFERENCES
bhakar, N. R. (2008). Induction of HIF-1 expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. Journal of Cellular Physiology, 217(3), 674–685. doi:10.1002/ jcp.21537.
40. Cai, Q., & Tammineni, P. (2017). Mitochondrial aspects of synaptic dysfunction in Alzheimer’s disease. Journal of Alzheimer’s Disease, 57(4), 1087–1103. doi:10.3233/ JAD-160726.
41. Koopman, W. J. H., Willems, P. H. G. M., & Smeitink, J. A. M. (2012). Monogenic mitochondrial disorders. New England Journal of Medicine, 366(12), 1132–1141. doi:10.1056/NEJMra1012478.
42. Norat, P., Soldozy, S., Sokolowski, J. D., Gorick, C. M., Kumar, J. S., Chae, Y., Yağmurlu, K., Prada, F., Walker, M., Levitt, M. R., Price, R. J., Tvrdik, P., & Kalani, M. Y. S. (2020). Mitochondrial dysfunction in neurological disorders: exploring mitochondrial transplantation. NPJ Regenerative Medicine, 5(1), Article 1. doi:10.1038/ s41536-020-00107-x.
43. Zhao, X.-Y., Lu, M.-H., Yuan, D.-J., Xu, D.-E., Yao, P.-P., Ji, W.-L., Chen, H., Liu, W.-L., Yan, C.-X., Xia, Y.-Y., Li, S., Tao, J., & Ma, Q.-H. (2019). Mitochondrial dysfunction in neural injury. Frontiers in Neuroscience, 13, 30. doi:10.3389/fnins.2019.00030.
44. Huang, W.-J., Zhang, X., & Chen, W.-W. (2016). Role of oxidative stress in Alzheimer’s disease. Biomedical Reports, 4(5), 519–522. doi:10.3892/br.2016.630.
45. Johnson, K. A., Fox, N. C., Sperling, R. A., & Klunk, W. E. (2012). Brain imaging in Alzheimer disease. Cold Spring Harbor Perspectives in Medicine, 2(4). doi:10.1101/cshperspect.a006213.
46. Zhang, F., Niu, L., Li, S., & Le, W. (2019). Pathological impacts of chronic hypoxia on Alzheimer’s disease. ACS Chemical Neuroscience, 10(2), 902–909. doi:10.1021/ acschemneuro.8b00442.
47. Sprick, J. D., Mallet, R. T., Przyklenk, K., & Rickards, C. A. (2019). Ischaemic and hypoxic conditioning: potential for protection of vital organs. Experimental Physiology, 104(3), 278–294. doi:10.1113/EP087122.
48. Rybnikova, E. A., Nalivaeva, N. N., Zenko, M. Y., & Baranova, K. A. (2022). Intermittent hypoxic training as an effective tool for increasing the adaptive potential, endurance and working capacity of the brain. Frontiers in Neuroscience, 16. doi:10.3389/fnins.2022.941740.
A Glitch in the Mental Matrix: Exploring the Illusion of déjà Vu
1. Engel, J. (2001). Mesial temporal lobe epilepsy: what have we learned? Neuroscientist, 7(4), 340-352. doi:10.1177/107385840100700410.
2. Stafstrom, C. E., & Carmant, L. (2015). Seizures and epilepsy: an overview for neuroscientists. Cold Spring
Harbor Perspectives in Medicine, 5(6). doi:10.1101/cshperspect.a022426.
3. Hine, K., & Tsushima, Y. (2018). Not explicit but implicit memory is influenced by individual perception style. PLoS One, 13(1), doi:10.1371/journal.pone.0191654.
4. Brown, S., & Craik, F. (2000). Encoding and Retrieval of Information. In Craik, F. The Oxford Handbook of Memory, pp. 93-107. Oxford University Press.
5. Yonelinas, A. P. (2002). The nature of recollection and familiarity: a review of 30 years of research. Journal of Memory and Language, 46(3), 441-517. doi:10.1006/ jmla.2002.2864.
6. Cleary, A. M. (2008). Recognition, memory, familiarity, and déjà vù experiences. Current Directions in Psychological Science, 17(5), 353-357. doi:10.1111/j.14678721.2008.00605.x.
7. Squire, L. R., Wixted, J. T., & Clark, R. E. (2007). Recognition memory and the medial temporal lobe: a new perspective. Nature reviews. Neuroscience, 8(11), 872–883. doi:10.1038/nrn2154.
8. Wild, E. (2005). déjà vù in neurology. Journal of Neurology, 252, 1–7. doi:10.1007/s00415-005-0677-3.
9. Illman, N. A., Butler, C. R., Souchay, C., & Moulin, C. J. (2012). déjà experiences in temporal lobe epilepsy. Epilepsy research and treatment, 2012, 539567. doi:10.1155/2012/539567.
10. Patel, A., Biso, G. M. N. R., & Fowler, J. B. (2022). Neuroanatomy, Temporal Lobe. In StatPearls. StatPearls Publishing. PMID:30137797.
11. Kodankandath, T. V., Theodore, D., & Samanta, D. (2022). Generalized tonic-clonic seizure. In StatPearls. StatPearls Publishing. PMID:32119383.
12. Petitmengin P., Baulac, M., & Navarro, V. (2006). Seizure anticipation: are neurophenomenological approaches able to detect preictal symptoms?. Epilepsy & Behavior, 9(2), 298-306. doi:10.1016/j.yebeh.2006.05.013.
13. Cleary, A. M., Neisser, J., McMahan, T., Parsons, T. D., Alwaki, A., Okada, N., Vosoughi, A., Kheder, A., Drane, D. L., & Pedersen, N. P. (2021). Subjective distinguishability of seizure and non-seizure déjà vu: a case report, brief literature review, and research prospects. Epilepsy & behavior: E&B, 125, 108373. doi:10.1016/j.yebeh.2021.108373.
14. Bancaud, J., Brunet-Bourgin, F., Chauvel, P., & Halgren, E. (1994). Anatomical origin of déjà vù and vivid “memories” in human temporal lobe epilepsy. Brain, 117(1), 71–90. doi:10.1093/brain/117.1.71.
15. Vignal, J., Maillard, L., McGonigal, A., & Chauvel, P. (2007). The dreamy state: hallucinations of autobiographic memory evoked by temporal lobe stimulations and seizures. Brain, 130(1), 88–99. doi:10.1093/brain/awl329.
16. Gillinder, L., Liegeois-Chauvel, C., & Chauvel P. (2022). What déjà vù and the “dreamy state” tell us about episodic memory networks. Clinical Neurophysiology, 136, 173-181. doi:10.1016/j.clinph.2022.01.126.
GREY MATTERS JOURNAL AT VASSAR COLLEGE | ISSUE 6 54
REFERENCES
17. Ter Meulen, B., Van der Meer, M., Hemmes, R. & Blom, J. D. (2021). déjà Vécu: When Groundhog Day Gets Real. Archives of Clinical and Medical Case Reports, 5. doi: 10.26502/acmcr.96550335.
18. Labate, A., Cerasa, A., Mumoli, L., Ferlazzo, E., Aguglia, U., Quattrone, A., & Gambardella, A. (2015). Neuro-anatomical differences among epileptic and non-epileptic déjà-vu. Cortex, 64, 1-7. doi:10.1016/j. cortex.2014.09.020.
19. Brázdil, M., Mareček, R., Urbánek, T., Kašpárek, T., Mikl, M., Rektor, I., & Zeman, A. (2021). Unveiling the mystery of déjà vù: the structural anatomy of déjà vù. Cortex, 48(9), 1240-1243, doi:10.1016/j.cortex.2012.03.004.
20. Irish, M., Hornberger, M., El Wahsh, S., Lam, B. Y., Lah, S., Miller, L., Hsieh, S., Hodges, J. R., & Piguet, O. (2014). Grey and white matter correlates of recent and remote autobiographical memory retrieval - insights from the dementias. PLoS One, 9(11). doi:10.1371/journal.pone.0113081.
21. Li, X., Wang, H., Tian, Y., Zhou, S., Li, X., Wang, K., & Yu, Y. (2016). Impaired white matter connections of the limbic system networks associated with impaired emotional memory in Alzheimer’s disease. Frontiers in Aging Neuroscience, 8, 250. doi:10.3389/ fnagi.2016.00250.
22. Maguire, E. A., Woollett, K., & Spiers, H. J. (2006). London taxi drivers and bus drivers: a structural MRI and neuropsychological analysis. Hippocampus, 16(12), 1091–1101. doi:10.1002/hipo.20233.
23. Rolls E. T. (2019). The cingulate cortex and limbic systems for emotion, action, and memory. Brain Structure & Function, 224(9), 3001–3018. doi:10.1007/ s00429-019-01945-2.
24. Zarrindast, M. R., & Khakpai, F. (2020). State-dependent memory and its modulation by different brain areas and neurotransmitters. EXCLI Journal, 19, 1081–1099. doi:10.17179/excli2020-2612.
25. O’Connor, A. R., & Moulin, C. J. A. (2008). The persistence of erroneous familiarity in an epileptic male: challenging perceptual theories of déjà vù activation. Brain and Cognition, 68(2), 144-147, doi:10.1016/j. bandc.2008.03.007.
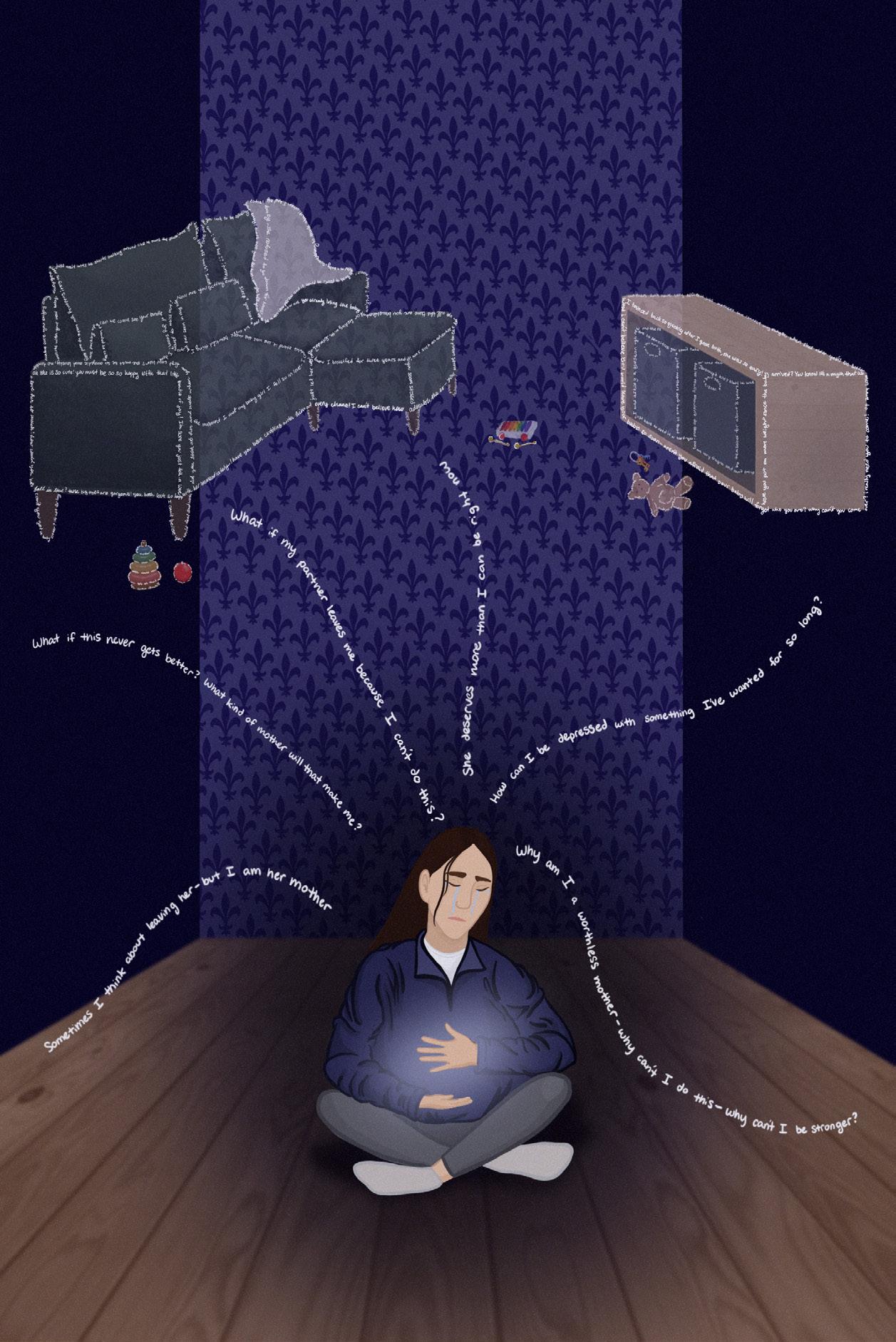
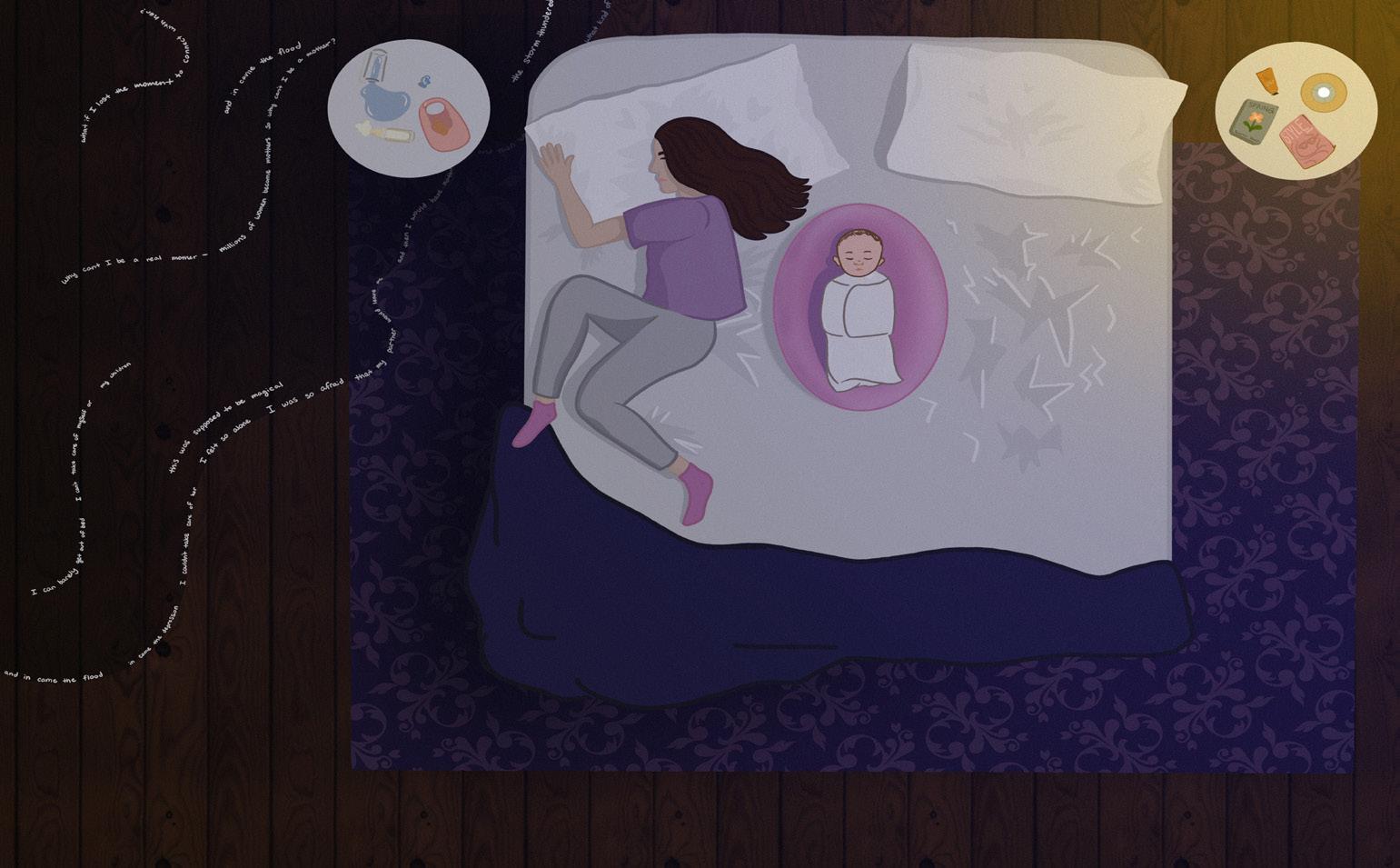
Beyond Baby Blues: Unpacking Postpartum Depression
1. Thomas, L. J., Scharp, K. M., & Paxman, C. G. (2014). Stories of postpartum depression: Exploring health constructs and help-seeking in mothers’ talk. Women & Health, 54(4), 373-387. doi:10.1080/03630242.2014.8 96442.
2. Romano, M., Cacciatore, A., Giordano, R., & La Rosa, B. (2010). Postpartum period: three distinct but continuous phases. Journal of Prenatal Medicine. 4(2), 22-5. PMID: 22439056.
3. Lopez-Gonzalez, D. M., & Kopparapu, A. K. (2020). Postpartum care of the new mother. PMID: 33351433.
4. Workman, J. L., Barha, C. K., & Galea, L. A. M. (2012). Endocrine substrates of cognitive and affective changes during pregnancy and postpartum. Behavioral Neuroscience, 126(1), 54–72. doi:10.1037/a0025538.
5. Cárdenas, E. F., Kujawa, A., Humphreys, K. L. (2020). Neurobiological changes during the peripartum period: implications for health and behavior, Social Cognitive and Affective Neuroscience, 15(10), 1097–1110. doi:10.1093/ scan/nsz091.
6. Mughal, S., Azhar, Y., & Siddiqui, W. (2022). Postpartum depression. In StatPearls [Internet]. StatPearls Publishing. PMID:30085612.
7. Olza, I., Uvnas-Moberg, K., Ekström-Bergström, A., Leahy-Warren, P., Karlsdottir, S. I., Nieuwenhuijze, M., Villarmea, S., Hadjigeorgiou, E., Kazmierczak, M., Spyridou, A., & Buckley, S. (2020). Birth as a neuro-psycho-social event: An integrative model of maternal experiences and their relation to neurohormonal events during childbirth. Plos one, 15(7). doi:10.1371/journal.pone.0230992.
8. Li, H., Bowen, A., Bowen, R., Balbuena, L., Feng, C., Bally, J., Muhajarine, N. (2020). Mood instability during pregnancy and postpartum: a systematic review. Archives of Women’s Mental Health, 23, 29–41. doi:10.1007/s00737019-00956-6.
9. Degner, D. (2017). Differentiating between “baby blues,” severe depression, and psychosis. BMJ, 359. doi:10.1136/ bmj.j4692.
10. American Psychiatric Association. (2000). Mood Disorders. In Diagnostic and Statistical Manual of Mental Disorders, (4th ed., text rev.). doi:10.1176/appi. books.9780890423349
11. Otte, C., Gold, S., Penninx, B., Pariante, C. M., Etkin, A., Maurizio, F., Mohr, D. C., Schatzberg, A. F. (2016). Major depressive disorder. Nature Reviews Disease Primers, 2, 16065. doi:10.1038/nrdp.2016.65.
12. Pan, Z., Park, C., Brietzke, E., Zuckerman, H., Rong, C., Mansur, R., Fus, D., Subramaniapillai, M., Lee, Y., McIntyre, R. (2019). Cognitive impairment in major depressive disorder. CNS Spectrums, 24(1), 22-29. doi:10.1017/ S1092852918001207.
13. Rai, S., Pathak, A., & Sharma, I. (2015). Postpartum psychiatric disorders: Early diagnosis and management. Indian Journal of Psychiatry, 57(Suppl 2), S216. doi:10.4103/0019-5545.161481.
14. Sharma, V., & Sharma, P. (2012). Postpartum depression: diagnostic and treatment issues. Journal of Obstetrics and Gynaecology Canada, 34(5), 436-442. doi:10.1016/ S1701-2163(16)35240-9.
15. O’Hara, M. W., & Wisner, K. L. (2014). Perinatal mental illness: definition, description and aetiology. Best practice & research Clinical obstetrics & gynaecology, 28(1), 3-12. doi:10.1016/j.bpobgyn.2013.09.002.
GREY MATTERS JOURNAL AT VASSAR COLLEGE | ISSUE 6 55 REFERENCES
16. Slomian J., Honvo G., Emonts P., Reginster J. Y., Bruyère O. (2019). Consequences of maternal postpartum depression: A systematic review of maternal and infant outcomes. Women’s Health, 15. doi:10.1177/1745506519844044.
17. Jacques, N., de Mola, C. L., Joseph, G., Mesenburg, M. A., & da Silveira, M. F. (2019). Prenatal and postnatal maternal depression and infant hospitalization and mortality in the first year of life: a systematic review and meta-analysis. Journal of affective disorders, 243, 201-208. doi:10.1016/j.jad.2018.09.055.
18. Farías-Antúnez, S., Xavier, M. O., & Santos, I. S. (2018). Effect of maternal postpartum depression on offspring’s growth. Journal of affective disorders, 228, 143-152. doi:10.1016/j.jad.2017.12.013.
19. O’hara, M. W., & McCabe, J. E. (2013). Postpartum depression: current status and future directions. Annual review of clinical psychology, 9, 379-407. doi:10.1146/annurev-clinpsy-050212-185612.
20. Liu, X., Wang, S., & Wang, G. (2022). Prevalence and Risk Factors of Postpartum Depression in Women: A Systematic Review and Meta-analysis. Journal of Clinical Nursing, 31, 2665– 2677. doi:10.1111/jocn.16121.
21. Vigod, S., Villegas, L., Dennis, C.-L. and Ross, L. (2010). Prevalence and risk factors for postpartum depression among women with preterm and low-birth-weight infants: a systematic review. BJOG: An International Journal of Obstetrics & Gynaecology, 117, 540-550. doi:10.1111/ j.1471-0528.2009.02493.x.
22. Urbanová, E.; Škodová, Z.; Bašková, M. (2021). The association between birth satisfaction and the risk of postpartum depression. International Journal of Environmental Research and Public Health, 18, 10458. doi:10.3390/ijerph181910458.
23. Bell, A. F., & Andersson, E. (2016). The birth experience and women’s postnatal depression: A systematic review. Midwifery, 39, 112-123. doi:10.1016/j.midw.2016.04.014.
24. Payne, J. L., & Maguire, J. (2019). Pathophysiological mechanisms implicated in postpartum depression. Frontiers in Neuroendocrinology, 52, 165-180. doi:10.1016/j. yfrne.2018.12.001.
25. Chambers, B. D., Arabia, S. E., Arega, H. A., Altman, M. R., Berkowitz, R., Feuer, S. K., Franck, L. S., Gomez, A. M., Kober, K., Pacheco-Werner, T., Paynter, R. A., Prather, A. A., Spellen, S. A., Stanley, D., Jelliffe-Pawlowski, L. L., McLemore, M. R. (2020). Exposures to structural racism and racial discrimination among pregnant and early post-partum Black women living in Oakland, California. Stress Health, 36 213– 219. doi:10.1002/smi.2922.
26. Rodriguez, A. C. I., Polcari, J. J., Nephew, B. C., Harris, R., Zhang, C., Murgatroyd, C., & Santos Jr, H. P. (2022). Acculturative stress, telomere length, and postpartum depression in Latinx mothers. Journal of Psychiatric Research, 147, 301-306. doi:10.1016/j.jpsychires.2022.01.063.
27. Alegría, M., Chatterji, P., Wells, K., Cao, Z., Chen, C. N., Takeuchi, D., Jackson, J., & Meng, X. L. (2015). Disparity in depression treatment among racial and ethnic minority populations in the United States. Psychiatric Services, 59(11), 1264-1272. doi:10.1176/ps.2008.59.11.1264.
28. Alang, SM (2019). Mental health care among blacks in America: Confronting racism and constructing solutions. Health Services Research. 54, 346– 355. doi:10.1111/14756773.13115.
29. Yu, Y., Liang, H. F., Chen, J., Li, Z. B., Han, Y. S., Chen, J. X., & Li, J. C. (2021). Postpartum depression: Current status and possible identification using biomarkers. Frontiers in Psychiatry, 12, 620371. doi:10.3389/fpsyt.2021.620371.
30. Berger, A. (2002). Magnetic resonance imaging. BMJ. 324(7328), 35. doi:10.1136/bmj.324.7328.35.
31. Chow, M. S., Wu, S. L., Webb, S. E., Gluskin, K., & Yew, D. T. (2017). Functional magnetic resonance imaging and the brain: A brief review. World Journal of Radiology, 9(1), 5. doi:10.4329/wjr.v9.i1.5.
32. Horáková, A., Němcová, H., Mohr, P., & Sebela, A. (2022). Structural, functional, and metabolic signatures of postpartum depression: A systematic review. Frontiers in Psychiatry, 13. doi:10.3389/fpsyt.2022.1044995.
33. Zhou, Y., Danbolt, N.C. (2014). Glutamate as a neurotransmitter in the healthy brain. Journal of Neural Transmission, 121, 799–817. doi:10.1007/s00702-014-1180-8.
34. Peng, S., Zhang, Y., Zhang, J., Wang, H., & Ren, B. (2011). Glutamate receptors and signal transduction in learning and memory. Molecular Biology Reports, 38, 453–460. doi:10.1007/s11033-010-0128-9.
35. Onaolapo, A. Y., & Onaolapo, O. J. (2021). Glutamate and depression: Reflecting a deepening knowledge of the gut and brain effects of a ubiquitous molecule. World Journal of Psychiatry, 11(7), 297. doi:10.5498/wjp.v11. i7.297.
36. Li, C. T., Yang, K. C., & Lin, W. C. (2019). Glutamatergic dysfunction and glutamatergic compounds for major psychiatric disorders: evidence from clinical neuroimaging studies. Frontiers in psychiatry, 9, 767. doi:10.3389/ fpsyt.2018.00767.
37. De Mayo, F. J., Zhao, B., Takamoto, N., & Tsai, S. Y. (2002). Mechanisms of action of estrogen and progesterone. Annals of the New York Academy of Sciences, 955(1), 48-59. doi:10.1111/j.1749-6632.2002.tb02765.x.
38. Schiller, C., Meltzer-Brody, S., & Rubinow, D. (2015). The role of reproductive hormones in postpartum depression. CNS Spectrums, 20(1), 48-59. doi:10.1017/ S1092852914000480.
39. McIntyre, K. R., Vincent, K. M., Hayward, C. E., Li, X., Sibley, C. P., Desforges, M., Greenwood, S. L., & Dilworth, M. R. (2020). Human placental uptake of glutamine and glutamate is reduced in fetal growth restriction. Scientific Reports, 10(1), 16197. doi:10.1038/s41598-020-72930-7.
40. Tsesis, S., Gruenbaum, B. F., Ohayon, S., Boyko, M., Gru-
GREY MATTERS JOURNAL AT VASSAR COLLEGE | ISSUE 6 56 REFERENCES
enbaum, S. E., Shapira, Y., Weintraub, A., & Zlotnik, A. (2013). The effects of estrogen and progesterone on blood glutamate levels during normal pregnancy in women. Gynecological Endocrinology, 29(10), 912-916. doi:10.3109/09513590.2013.813467.
41. Barth, C., Villringer, A., & Sacher, J. (2015). Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Frontiers in Neuroscience, 9(37). doi:10.3389/fnins.2015.00037.
42. McEwen, A. M., Burgess, D. T .A., Hanstock, S. E. C., Hanstock, C. C., Seres, P., Khalili, P., Newman, S. C., Baker, G. B., Mitchell, N. D., Allen, P. S. & Le Melledo, J. M. (2021). Glutamate levels in the medial prefrontal cortex of healthy pregnant women compared to non-pregnant controls. Psychoneuroendocrinology, 133, 105382. doi:10.1016/j.psyneuen.2021.105382.
43. Ghuman, A., McEwen, A., Tran, K. H., Mitchell, N., Hanstock, C., Seres, P., Jhangri, G., Burgess, D., Baker, G., & Melledo, J. M. L. (2022). Prospective investigation of glutamate levels and percentage gray matter in the medial prefrontal cortex in females at risk for postpartum depression. Current Neuropharmacology, 20(10), 1988-2000. doi:10.2174/1570159X20666220302101115.
44. Smith, R., Lane, R. D., Alkozei, A., Bao, J., Smith, C., Sanova, A., Nettles, M., & Killgore, W. D. (2018). The role of medial prefrontal cortex in the working memory maintenance of one’s own emotional responses. Scientific Reports, 8(1), 1-15. doi:10.1038/s41598-018-21896-8.
45. Xu, P., Chen, A., Li, Y., Xing, X., & Lu, H. (2019). Medial prefrontal cortex in neurological diseases. Physiological genomics, 51(9), 432-442. doi:/10.1152/physiolgenomics.00006.2019.
46. Rosa, C. E., Soares, J. C., Figueiredo, F. P., Cavalli, R. C., Barbieri, M. A., Schaufelberger, M. S., Salmon, C. E. G., Del-Ben, C. M., & Santos, A. C. (2017). Glutamatergic and neural dysfunction in postpartum depression using magnetic resonance spectroscopy. Psychiatry Research: Neuroimaging, 265, 18-25. doi:10.1016/j.pscychresns.2017.04.008.
47. Hertrich, I., Dietrich, S., Blum, C., & Ackermann, H. (2021). The role of the dorsolateral prefrontal cortex for speech and language processing. Frontiers in Human Neuroscience, 15, 645209. doi:10.3389/fnhum.2021.645209.
48. Fairless, R., Bading, H., & Diem, R. (2021). Pathophysiological ionotropic glutamate signaling in neuroinflammatory disease as a therapeutic target. Frontiers in Neuroscience, 15(741280). doi:10.3389/ fnins.2021.741280.
49. van Beek, E. J. R., Kuhl, C., Anzai, Y., Desmond, P., Ehman, R. L., Gong, Q., Gold, G., Gulani, V., Hall-Craggs, M., Leiner, T., Lim, C. C. T., Pipe, J. G., Reeder, S., Reinhold, C., Smits, M., Sodickson, D. K., Tempany, C., Vargas, H. A., & Wang, M. (2019). Value of MRI in med-
REFERENCES
icine: More than just another test?. Journal Magnetic Resonance Imaging, 49, e14-e25. doi:10.1002/jmri.26211.
50. Duan, C., Cosgrove, J., & Deligiannidis, K. M. (2017). Understanding peripartum depression through neuroimaging: a review of structural and functional connectivity and molecular imaging research. Current psychiatry reports, 19(70), 1-13. doi:10.1007/s11920-017-0824-4.
51. Cheng, B., Zhou, Y., Kwok, V. P., Li, Y., Wang, S., Zhao, Y., Meng, Y., Deng, W., & Wang, J. (2022). Altered functional connectivity density and couplings in postpartum depression with and without anxiety. Social Cognitive and Affective Neuroscience, 17(8), 756-766. doi:10.1093/scan/ nsab127.
52. Rolls, E. T. (2019). The cingulate cortex and limbic systems for emotion, action, and memory. Brain Structure and Function, 224(9), 3001-3018. doi:10.1007/s00429019-01945-2.
53. Gallagher, M., & Chiba, A. A. (1996). The amygdala and emotion. Current Opinion in Neurobiology, 6(2), 221-227. doi:10.1016/S0959-4388(96)80076-6.
54. Meisner, O. C., Nair, A., & Chang, S. W. (2022). Amygdala connectivity and implications for social cognition and disorders. In Handbook of Clinical Neurology, 187, 381403. doi:10.1016/B978-0-12-823493-8.00017-1.
55. Chase, H. W., Moses-Kolko, E. L., Zevallos, C., Wisner, K. L., & Phillips, M. L. (2014). Disrupted posterior cingulate–amygdala connectivity in postpartum depressed women as measured with resting BOLD fMRI. Social Cognitive and Affective Neuroscience, 9(8), 1069-1075. doi:10.1093/ scan/nst083.
56. Laurent, H. K., & Ablow, J. C. (2013). The missing link: mothers’ neural response to infant cry related to infant attachment behaviors. Infant Behavior and Development, 35(4), 761-772. doi:10.1016/j.infbeh.2012.07.007.
57. Laurent, H. K., & Ablow, J. C. (2012). A cry in the dark: depressed mothers show reduced neural activation to their own infant’s cry. Social Cognitive and Affective Neuroscience, 7(2), 125-134. doi:10.1093/scan/nsq091.
58. Frieder, A., Fersh, M., Hainline, R., & Deligiannidis, K. M. (2019). Pharmacotherapy of postpartum depression: current approaches and novel drug development. CNS Drugs, 33, 265-282. doi:10.1007/s40263-019-00605-7.
59. Hofmann, S. G., Curtiss, J., Carpenter, J. K., & Kind, S. (2017). Effect of treatments for depression on quality of life: a meta-analysis. Cognitive Behavior Therapy, 46(4), 265-286. doi:10.1080/16506073.2017.1304445.
60. Lochmann, D., & Richardson, T. (2019). Selective serotonin reuptake inhibitors. Antidepressants: From biogenic amines to new mechanisms of action, 135-144. doi:10.1007/164_2018_172.
61. Viswanathan, M., Middleton, J. C., Stuebe, A. M., Berkman, N. D., Goulding, A. N., McLaurin‐Jiang, S., Dotson, A. B., Coker-Schwimmer, M., Baker, C., Voisin, C. E., Bann, C., & Gaynes, B. N. (2021). Maternal, fetal, and
GREY MATTERS JOURNAL AT VASSAR COLLEGE | ISSUE 6 57
child outcomes of mental health treatments in women: a meta‐analysis of pharmacotherapy. Psychiatric Research and Clinical Practice, 3(3), 123-140. doi:10.1176/ appi.prcp.20210001.
62. Oberlander, T. F., Warburton, W., Misri, S., Aghajanian, J., & Hertzman, C. (2006). Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Archives of General Psychiatry, 63(8), 898-906. doi:10.1001/archpsyc.63.8.898.
63. Malm, H., Sourander, A., Gissler, M., Gyllenberg, D., Hinkka-Yli-Salomäki, S., McKeague, I. W., Artama, M., & Brown, A. S. (2015). Pregnancy complications following prenatal exposure to SSRIs or maternal psychiatric disorders: results from population-based national register data. American Journal of Psychiatry, 172(12), 1224-1232. doi:10.1176/appi.ajp.2015.14121575.
64. De Crescenzo, F., Perelli, F., Armando, M., & Vicari, S. (2014). Selective serotonin reuptake inhibitors (SSRIs) for postpartum depression (PPD): a systematic review of randomized clinical trials. Journal of Affective Disorders, 152, 39-44. doi:10.1016/j.jad.2013.09.019.
65. Thomson, M., & Sharma, V. (2017). Therapeutics of postpartum depression. Expert Review of Neurotherapeutics, 17(5), 495-507. doi:10.1080/14737175.2017.1265888
66. Marchocki, Z., Russell, N. E., & Donoghue, K. O. (2013). Selective serotonin reuptake inhibitors and pregnancy: A review of maternal, fetal and neonatal risks and benefits. Obstetric Medicine, 6(4), 155-158. doi:10.1177/1753495X13495194.
67. Sockol, L. E. (2015). A systematic review of the efficacy of cognitive behavioral therapy for treating and preventing perinatal depression. Journal of Affective Disorders, 177, 7-21. doi:10.1016/j.jad.2015.01.052.
68. Thoma, N., Pilecki, B., & McKay, D. (2015). Contemporary cognitive behavior therapy: A review of theory, history, and evidence. Psychodynamic Psychiatry, 43(3), 423461. doi:10.1521/pdps.2015.43.3.423.
69. Bigelow, A. E., & Power, M. (2020). Mother–infant skinto-skin contact: short‐and long-term effects for mothers and their children born full-term. Frontiers in Psychology, 11, 1921. doi:10.3389/fpsyg.2020.01921.
70. Widström, A. M., Brimdyr, K., Svensson, K., Cadwell, K., & Nissen, E. (2019). Skin‐to‐skin contact the first hour after birth, underlying implications and clinical practice. Acta Paediatrica, 108(7), 1192-1204. doi:10.1111/apa.14754.
71. Campbell-Yeo, M. L., Disher, T. C., Benoit, B. L., & Johnston, C. C. (2015). Understanding kangaroo care and its benefits to preterm infants. Pediatric Health, Medicine and Therapeutics, 15-32. doi:10.2147/PHMT.S51869.
72. Cooijmans, K. H., Beijers, R., Rovers, A. C., & de Weerth, C. (2017). Effectiveness of skin-to-skin contact versus care-as-usual in mothers and their full-term infants: study protocol for a parallel-group randomized con-
trolled trial. BMC Pediatrics, 17, 1-16. doi:10.1186/s12887017-0906-9.
73. Badr, H. A., & Zauszniewski, J. A. (2017). Kangaroo care and postpartum depression: The role of oxytocin. International Journal of Nursing Sciences, 4(2), 179-183. doi:10.1016/j.ijnss.2017.01.001.
74. Wisner, K. L., Moses-Kolko, E. L., & Sit, D. K. (2010). Postpartum depression: a disorder in search of a definition. Archives of Women’s Mental Health, 13, 37-40. doi:10.1007/s00737-009-0119-9.
75. Jolley, S. N., & Betrus, P. (2007). Comparing postpartum depression and major depressive disorder: issues in assessment. Issues in Mental Health Nursing, 28(7), 765780. doi:10.1080/01612840701413590.
76. Spinelli, M. (2021). Postpartum psychosis: a diagnosis for the DSM V. Archives of Women’s Mental Health, 1-6. doi:10.1007/s00737-021-01175-8.
77. American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). doi:10.1176/appi.books.9780890425596
78. Schanie, C. L., Pinto-Foltz, M. D., & Logsdon, M. C. (2008). Analysis of popular press articles concerning postpartum depression: 1998–2006. Issues in Mental Health Nursing, 29(11), 1200-1216. doi:10.1080/01612840802370509.
79. Martinez, R., Johnston-Robledo, I., Ulsh, H. M., & Chrisler, J. C. (2001). Singing “the baby blues:” A content analysis of popular press articles about postpartum affective disturbances. Women & Health, 31(2-3), 37-56. doi:10.1300/J013v31n02_02.
80. Hesse, B. W., Nelson, D. E., Kreps, G. L., Croyle, R. T., Arora, N. K., Rimer, B. K., & Viswanath, K. (2005). Trust and sources of health information: the impact of the Internet and its implications for health care providers: findings from the first Health Information National Trends Survey. Archives of Internal Medicine, 165(22), 2618-2624. doi:10.1001/archinte.165.22.2618.
81. Jacobs, W., Amuta, A. O., & Jeon, K. C. (2017). Health information seeking in the digital age: An analysis of health information seeking behavior among US adults. Cogent Social Sciences, 3(1), 1302785. doi:10.1080/23311 886.2017.1302785.
82. Coyne, S. M., Liechty, T., Collier, K. M., Sharp, A. D., Davis, E. J., & Kroff, S. L. (2018). The effect of media on body image in pregnant and postpartum women. Health Communication, 33(7), 793-799. doi:10.1080/10410236.2 017.1314853.
83. Han, J. W., & Kim, D. J. (2020). Longitudinal relationship study of depression and self-esteem in postnatal Korean women using autoregressive cross-lagged modeling. International Journal of Environmental Research and Public Health, 17(10), 3743. doi:10.3390/ijerph17103743.
84. Frankhouser, T. L., & Defenbaugh, N. L. (2017). An autoethnographic examination of postpartum depression. The Annals of Family Medicine, 15(6), 540-545.
GREY MATTERS JOURNAL AT VASSAR COLLEGE | ISSUE 6 58 REFERENCES
doi:10.1370/afm.2107.
85. Fleischman, E. K., Connelly, C. D., & Calero, P. (2022). Depression and Anxiety, Stigma, and Social Support Among Women in the Postpartum Period. Nursing for Women’s Health, 26(2), 95-106. doi:10.1016/j. nwh.2022.01.008.
86. Lin, P. Z., Xue, J. M., Yang, B., Li, M., & Cao, F. L. (2018). Effectiveness of self-help psychological interventions for treating and preventing postpartum depression: a meta-analysis. Archives of Women’s Mental Health, 21, 491-503. doi:10.1007/s00737-018-0835-0.
87. Knaak, S. (2009). “ Having a Tough Time:” Towards an Understanding of the Psycho-social Causes of Postpartum Emotional Stress. Journal of the Motherhood Initiative for Research and Community Involvement, 11, 80-94.
88. Corrigan, P. W., Watson, A. C., & Barr, L. (2006). The self–stigma of mental illness: Implications for self–esteem and self–efficacy. Journal of Social and Clinical Psychology, 25(8), 875-884. doi:10.1521/jscp.2006.25.8.875.
89. Thorsteinsson, E. B., Loi, N. M., & Farr, K. (2018). Changes in stigma and help-seeking in relation to postpartum depression: non-clinical parenting intervention sample. PeerJ, 6, e5893. doi:10.7717/peerj.5893
90. Dennis, C. L., & Chung‐Lee, L. (2006). Postpartum depression help‐seeking barriers and maternal treatment preferences: A qualitative systematic review. Birth, 33(4), 323-331. doi:10.1111/j.1523-536X.2006.00130.x.
91. Hadfield, H., & Wittkowski, A. (2017). Women’s experiences of seeking and receiving psychological and psychosocial interventions for postpartum depression: a systematic review and thematic synthesis of the qualitative literature. Journal of Midwifery & Women’s Health, 62(6), 723-736. doi:10.1111/jmwh.12669.
92. Wonch, K. E., de Medeiros, C. B., Barrett, J. A., Dudin, A., Cunningham, W. A., Hall, G. B., Steiner, M., & Fleming, A. S. (2016). Postpartum depression and brain response to infants: Differential amygdala response and connectivity. Social Neuroscience, 11(6), 600-617. doi:1 0.1080/17470919.2015.1131193.
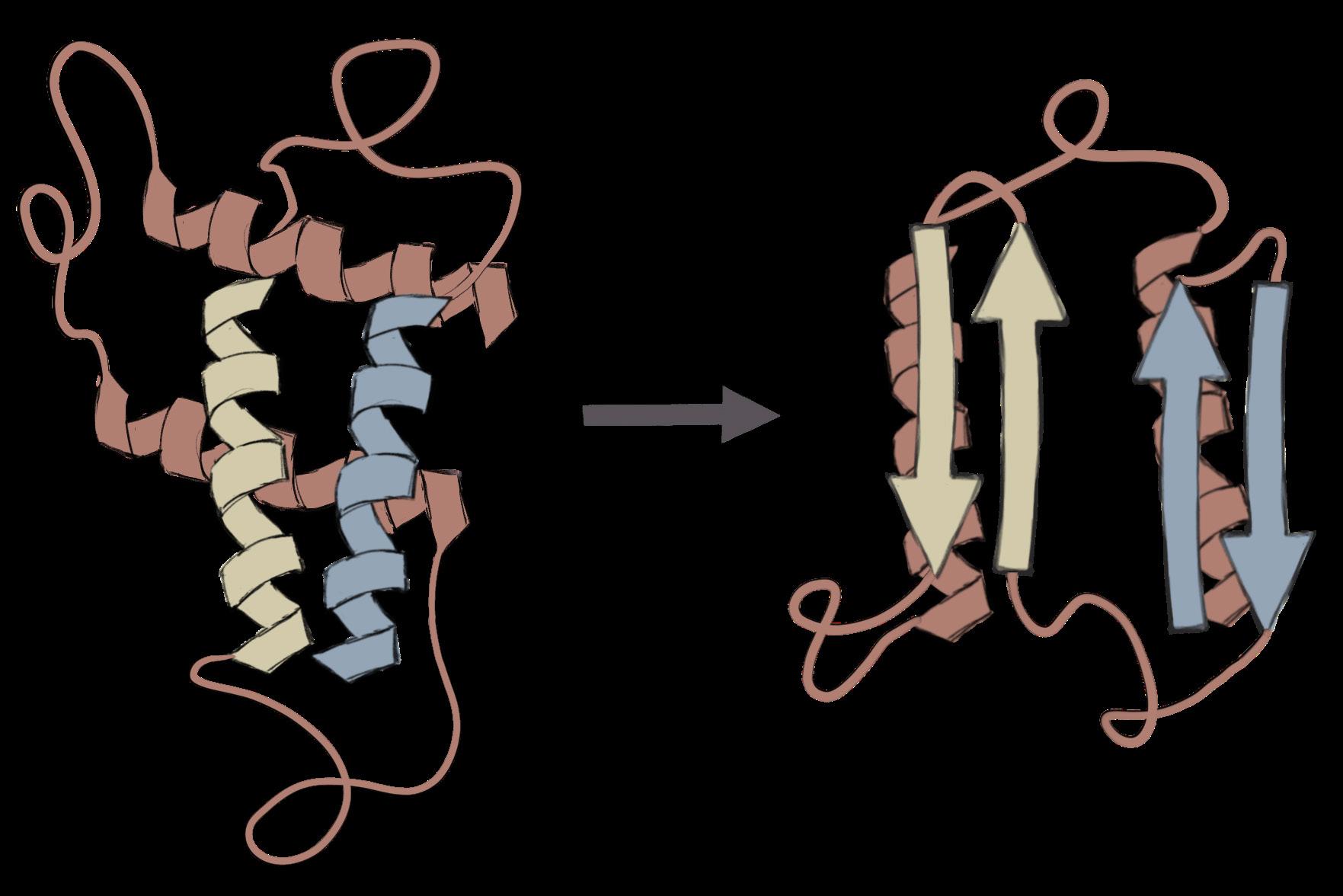
A Wrinkle in the Mind: How Prions Infect the Brain
1. Rentz, C. (2003). Creutzfeld-Jakob disease: Two case studies. American Journal of Alzheimer’s Disease and Other Dementias, 18(3). doi:10.1177/153331750301800309.
2. Sitammagari, K. K., & Masood, W. (2022). Creutzfeld-Jakob disease. StatPearls Publishing. PMID:29939637.
3. Brown, P. (2001). Bovine spongiform encephalopathy and variant Creutzfeld-Jakob disease. British Medical Journal, 322(7290), 841-844. doi:10.1136/ bmj.322.7290.841.
4. Chen, Y., Ding, F., Nie, H., Serohijos, A. W., Sharma, S.,
REFERENCES
Wilcox, K. C., Yin, S., & Dokholyan, N. V. (2008). Protein folding: then and now. Archives of Biochemistry and Biophysics, 469(1), 4-19. doi:10.1016/j.abb.2007.05.014.
5. Saá, P., Harris, D. A., & Cervenakova, L. (2016). Mechanisms of prion-induced neurodegeneration. Expert Reviews in Molecular Medicine, 18(5). doi:10.1017/erm.2016.8.
6. Soto, C., & Satani, N. (2011). The intricate mechanisms of neurodegeneration in prion diseases. Trends in Molecular Medicine, 17(1), 14-24. doi:10.1016/j.molmed.2010.09.001.
7. Wulf, M., Senatore, A., & Aguzzi, A. (2017). The biological function of the cellular prion protein: an update. BMC Biology, 15(34). doi:10.1186/s12915-017-0375-5.
8. Walker, L. C. (2018). Prion-like mechanisms in Alzheimer’s disease. Handbook of Clinical Neurology, 153, 303319. doi:10.1016/B978-0-444-63945-5.00016-7.
9. Ma, J., Gao, J., Wang, J., & Xie, A. (2019). Prion-like mechanisms in Parkinson’s disease. Frontiers in Neuroscience, 13(552). doi:10.3389/fnins.2019.00552.
10. Tabernero, C., Polo, J. M., Sevillano, M. D., Muñoz, R., Berciano, J., Cabello, A., Báez, B., Ricoy, J. R., Carpizo, R., Figols, J., Cuadrado, N., & Claveria, L. E. (2000). Fatal familial insomnia: clinical, neuropathological, and genetic description of a Spanish family. Journal of Neurology, Neurosurgery, & Psychiatry, 68(6). doi:10.1136/ jnnp.68.6.774.
11. Edgeworth, J. A., Gros, N., Alden, J., Joiner, S., Wadsworth, J. D., Linehan, J., Brandner, S., Jackson, G. S., Weissman, C., & Collinge, J. (2010). Spontaneous generation of mammalian prions. Proceedings of the National Academy of Sciences, 172(32), 14402-14406. doi:10.1073/ pnas.1004036107.
12. Ward, H. J. T., Everington, D., Cousens, S. N., Smith-Bathgate, B., Leitch, M., Cooper, S., Heath, C., Knight, R. S. G., Smith, P. G., & Will, R. G. (2005). Risk factors for variant Creutzfeldt-Jakob disease: a case-control study. Annals of Neurology, 59(1), 111-120. doi:10.1002/ana.20708.
13. Sandberg, M. K., Al-Doujaily, H., Sharps, B., Clarke, A. R., & Collinge, J. (2011). Prion propagation and toxicity in vivo occur in two distinct mechanistic phases. Nature, 470, 540-542. doi:10.1038/nature09768.
14. Colby, D. W., & Pruisner, S. B. (2011). Prions. Cold Springs Harbor Perspectives in Biology, 3(1). doi:cshperspect. a006833.
15. Castle, A. R., & Gill, A. C. (2017). Physiological functions of the cellular prion protein. Frontiers in Molecular Biosciences, 4. doi:10.3389/fmolb.2017.00019.
16. Malhotra, P., & Udgaonkar, J. B. (2016). How cooperative are protein folding and unfolding transitions? Protein Science, 25(11), 1924-1941. doi:10.1002/pro.3015.
17. Portman, J. J. (2010). Cooperativity and protein folding rates. Current Opinion in Structural Biology, 20(1), 11-15. doi:10.1016/j.sbi.2009.12.013.
18. Stöhr, J., Weinmann, N., Willie, H., Kaimann, T., Nagel-Steger, L., Birkmann, E., Panza, G., Pruisner, S. B.,
GREY MATTERS JOURNAL AT VASSAR COLLEGE | ISSUE 6 59
Eigen, M., & Reisner, D. (2008). Mechanisms of prion protein assembly into amyloid. Proceedings of the National Academy of the Sciences, 105(5), 2409-2414. doi:10.1073/ pnas.0712036105.
19. Rossi, M., Saverioni, D., Di Bari, M., Baiardi, S., Lemstra, A. W., Pirisinu, L., Capellari, S., Rozemuller, A., Nonno, R., & Parchi, P. (2017). Atypical Creutzfeldt-Jakob disease with Prp-amyloid plaques in white matter: molecular characterization and transmission to bank voles show the M1 strain signature. Acta Neuropathologica Communications, 5(87). doi:10.1186/s40478-017-0496-7.
20. Legname, G. (2017). Elucidating the function of the prion protein. PLOS Pathogens, 13(8). doi:10.1073/ pnas.0712036105.
21. O’Leary, T., & Wyllie, D. J. A. (2011). Neuronal homeostasis: time for a change? The Journal of Physiology, 589(20), 4811-4826. doi:10.1113/jphysiol.2011.210179.
22. Bremer, J., Baumann, F., Tiberi, C., Wessig, C., Heike, F., Schwarz, P., Steele, A. D., Toyka, K. V., Nave K., Weis, J., & Aguzzi, A. (2010). Axonal prion protein is required for peripheral myelin maintenance. Nature Neuroscience, 13, 310-318. doi:10.1038/nn.2483.
23. Nuvolone, M., Hermann, M., Sorce, S., Russo, G., Tiberi, C., Schwarz, P., Minikel, E., Sanoudou, D., Pelczar, P., & Aguzzi, A. (2016). Strictly co-isogenic C57BL/6J-Prnp-/mice: a rigorous resource for prion science. Journal of Experimental Medicine, 213(3), 313-327. doi:10.1084/ jem.20151610.
24. Parrie, L. E., Crowell, J. A. E., Telling, G. C., & Bessen, R. A. (2018). The cellular prion protein promotes olfactory sensory neuron survival and axon targeting during adult neurogenesis. Developmental Biology, 438(1), 2332. doi:10.1016/j.ydbio.2018.03.012.
25. Rambaran, R. N., & Serpell, L. C. (2008). Amyloid fibrils. Prion, 2(3), 112-117. doi:10.4161/pri.2.3.7488.
26. Iadanza, M. G., Jackson, M. P., Hewitt, E. W., Ranson, N. A., & Radford, S. E. (2018). A new era for understanding amyloid structures and disease. Nature Reviews Molecular Cellular Biology, 19, 755-773. doi:10.1038/s41580018-0060-8
27. Thody, S. A., Matthew, M. K., & Udgaonkar, J. B. (2018). Mechanism of aggregation and membrane interactions of prion disease. Biochimica et Biophysica ActaBiomembranes, 1860(9), 1927-1935. doi:10.1016/j.bbamem.2018.02.031.
28. Pepys, M. B. (2001). Pathogenesis, diagnosis, and treatment of systemic amyloidosis. Philosophical Transactions of the Royal Society B, 356(1406), 203-211. doi:10.1098/rstb.2000.0766.
29. Ragagnin, A., Wang, Q., Guillemain, A., Dole, S., Wilding, A., Demais, V., Royer, C., Haeberlé, A., Vitale, N., Gasman, S., Grant, N., & Bailly, Y. (2018). Prion proteins and neuronal death in the cerebellum. InterOpen. doi:10.5772/ intechopen.80701.
30. Russelakis-Carneiro, M., Hetz, C., Maundrell, K., & Soto, C. (2004). Prion replication alters the distribution of synaptophysin and caveolin 1 in the neuronal lipid rafts. American Journal of Pathology, 165(5), 1839-48. doi:10.5772/intechopen.80701.
31. Chakrabarti, O., & Hedge, R. S. (2009). Functional depletion of mahogunin by cytosolically exposed prion protein contributes to neurodegeneration. Cell, 137(6), 11361147. doi:10.1016/j.cell.2009.03.042.
32. Hwang, D., Lee, I. Y., Yoo, H., Gehlenborg, N., Cho, J., Petretis, B., Baxter, D., Pitstick R., Young, R., Spicer, D., Price, N. D., Hohmann, J. G., Dearmond, S. J., Carlson, G. A., & Hood, L. E. (2009). A systems approach to prion disease. Molecular Systems Biology, 5(252). doi:10.1038/ msb.2009.10.
33. Holman, R. C., Belay, E. D., Christensen, K. Y., Maddox, R. A., Minio, A. M., Folkema, A. M., Haberling, D. L., Hammett, T. A., Kochanek, K. D., Sejvar, J. J., & Schonberger, L. B. (2010). Human prion disease in the United States. PLoS One, 5(1). doi:10.1371/journal.pone.0008521.
34. Chen, C., & Dong, X. (2016). Epidemiological characteristics of human prion diseases. Infectious Diseases of Poverty, 5(47). doi:10.1186/s40249-016-0143-8.
35. Maddox, R. A., Person, M. K., Blevins, J. E., Abrams, J. Y., Appleby, B. S., Schonberger, L. B., & Belay, E. D. (2020). Neurology, 94(2). doi:10.1212/WNL.00000000000086810
36. National Highway Traffic Safety Administration. (2020). Fars Encyclopedia. FARS Encyclopedia.
37. Kovač, V., & Šerbec, V. Č. (2022). Prion protein: the many forms and faces. International Journal of Molecular Sciences, 23(3), 1232. doi:10.3390/ijms23031232.
38. Imran, M., & Mahmood, S. (2011). An overview of animal prion diseases. Virology Journal, 8(493). doi:10.1186/1743422X-8-493.
39. Tamgüney, G., Giles, K., Bouzamondo-Bernstein, E., Bosque, P. J., Miller, M. W., Safar, J., DeArmond, S. J., & Pruisner, S. B. (2006). Transmission of elk and deer prions to transgenic mice. Journal of Virology, 80(18), 9104-9114. doi:10.1128/JVI.00098-06.
40. Llewellyn, C. A., Hewitt, P. E., Knight, R. S. G., Amar, K. Cousens, S., & Mackenzie, J. (2004). Possible transmission of variant Creutzfeld-Jakob disease by blood transfusion. The Lancet, 363(9407), 417-421. doi:10.1016/ S0140-6736(04)15486-X.
41. Gough, K. C., & Maddison, B. C. (2010). Prion transmission: prion excretion and occurrence in the environment. Prion, 4, 275-282. doi:10.4161/pri.4.4.13678.
42. Mastrianni, J. A. (2010). The genetics of prion diseases. Genetics in Medicine, 12, 187-195. doi:10.1097/GIM. 0b013e3181cd7374.
43. Clift, K., Guthrie, K., Klee, E. W., Boczek, N., Cousin, M., Blackburn, P., & Atwal, P. (2016). Familial Creutzfelt-Jakob disease: case report and role of genetic counseling in post mortem testing. Prion, 10(6), 502-506. doi:10.108
GREY MATTERS JOURNAL AT VASSAR COLLEGE | ISSUE 6 60 REFERENCES
0/19336896.2016.1254858.
44. Eckland, T. E., Shikiya, R. A., & Bartz, J. C. (2018). Independent amplification of co-infected long incubation period low conversion efficiency prion strains. PLoS Pathogens, 14(10). doi:10.1371/journal.ppat.1007323.
45. Kübler, E., Oesech, B., Raeber, A. J. (2003). Diagnosis of prion diseases. British Medical Bulletin, 66(1), 267-279. doi:10.1093/bmb/66.1.267.
46. McDonnell, G., & Burke, P. (2003). The challenge of prion decontamination. Clinical Infectious Diseases, 36(9), 1152-1154. doi:10.1086/374668.
47. Bonda, D. J., Manjila, S., Mehndritta, P., Khan, F., Miller, B. R., Onwuzulike, K., Puoti, G., Cohenn, M. L., Schonberger, L. B., & Cali, I. (2016). Human prion diseases: surgical lessons learned from iatrogenic prion transmission. Neurological Focus, 4(1). doi:10.3171/2016.5. FOCUS15126.
48. Pritzkow, S., Gorski, D., Ramirez, F., & Soto, C. (2021). Prion dissemination through environment and medical practices: facts and risks for human health. Clinical Microbiology Reviews, 34(4). doi:10.1128/CMR.0005919.
49. Bennetti, F., & Legname, G. (2015). New insights into structural determinants of prion protein folding and stability. Prion, 9(2), 119-124. doi:10.1080/19336896.20 15.1022023.
50. Liberski, P. P., Gajos, A., Sikorska, B., & Lindenbaum, S. (2019). Kuru, the first human prion disease. Viruses, 11(3), 232. doi:10.3390/v11030232.
51. Whittfield, J. T., Pako, W. H., Collinge, J., & Alpers, M. P. (2008). Mortuary rites of the South Fore and kuru. Philosophical Transactions of the Royal Society B: Biological Sciences, 363(1510), 3721-3724. doi:10.1098/ rstb.2008.0074.
52. Liberski, P. P., Sikorski, B., Lindenbaum, S., Goldfarb, L. G., McLean, C., Hainfeller, J. A., & Brown, P. (2012). Kuru: genes, cannibals, and neuropathology. Journal of Neuropathology & Experimental Neurology, 71(2), 92-103. doi:10.1097/NEN.0b013e3182444efd.
53. Liberski, P. P. (2013). Kuru: a journey back in time from Papua New Guinea to the Neanderthals’ extinction. Pathogens, 2(3), 472-505. doi:10.3390/pathogens2030472
54. Gomez-Gutierrez, R., & Morales, R. (2020). The prion-like phenomenon in Alzheimer’s disease: evidence of pathology transmission in humans. PLoS Pathology, 16(10). doi:10.1371/journal.ppat.1009004.
55. Prusiner, S. B. (2001). Neurodegenerative diseases and prions. New England Journal of Medicine, 344, 15161526. doi:10.1056/NEJM200105173442006.
56. Lyketsos, C. G., Carrillo, M. C., Ryan, J. M., Khachaturian, A. S., Trzepacz, P., Amatniek, J., Cedarbaum, J., Brashear, R., & Miller, D. S. (2012). Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimer’s De-
ment, 7(5), 532-539. doi:10.1016/j.jalz.2011.05.2410.
57. Kouli, A., Torsney, K. M., & Kuan, W. (2018). Parkinson’s disease: etiology, neuropathology, and pathogenesis. Codon Publications. doi:10.15586/codonpublications. parkinsonsdisease.2018.ch1.
58. Yamaguchi, K., & Kuwata, K. (2018). Formation and properties of amyloid fibrils of prion protein. Biophysical Reviews, 10(2), 517-525. doi:10.1007/s12551-017-0377-0.
59. Jung, M. J., Pistolesi, D., & Panà, A. (2003). Prions, prion diseases and decontamination. Igiene e Sanita Pubblica, 59(5), 331-44. PMID:14981553.
60. Aguzzi, A., & Zhu, C. (2012). Five questions on prion disease. PLoS Pathogens, 8(5). doi:10.1371/journal. ppat.1002651.
Exploring the Possibilities of Life Without a Brain
1. Butterfield, N. J. (2007). Macroevolution and macroecology through deep time. Palaeontology, 50, 41–55. doi:10.1111/j.1475-4983.2006.00613.x.
2. Brunet, T., & King, N. (2017). The origin of animal multicellularity and cell differentiation. Developmental Cell, 43(2), 124–140. doi:10.1016/j.devcel.2017.09.016.
3. Erwin, D., Valentine, J., & Jablonski, D. (1997). The origin of animal body plans: Recent fossil finds and new insights into animal development are providing fresh perspectives on the riddle of the explosion of animals during the Early Cambrian. American Scientist, 85(2), 126–137.
4. Kandel, E. R., Koester, J., Mack, S., & Siegelbaum, S. (2021). Principles of Neural Science (6th ed.). McGraw Hill.
5. Roth G. (2015). Convergent evolution of complex brains and high intelligence. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 370(1684), 20150049. doi:10.1098/rstb.2015.0049.
6. Burnett, S. (2011). Perceptual worlds and sensory ecology. Nature Education Knowledge, 3(10):75.
7. Hickman, C. P. (2020). Integrated principles of zoology (18th ed.). McGraw-Hill Education.
8. Watanabe, H., Fujisawa, T., & Holstein, T. W. (2009). Cnidarians and the evolutionary origin of the nervous system. Development, Growth & Differentiation, 51(3), 167–183. doi:10.1111/j.1440-169X.2009.01103.x.
9. Arendt, D., Denes, A. S., Jékely, G., & Tessmar-Raible, K. (2008). The evolution of nervous system centralization. Philosophical Transactions of the Royal Society B: Biological Sciences, 363(1496), 1523-1528. doi:10.1098/ rstb.2007.2242.
10. Kelava, I., Rentzsch, F., & Technau, U. (2015). Evolution of eumetazoan nervous systems: insights from cnidarians. Philosophical Transactions of the Royal Society B: Biological Sciences, 370. doi:10.1098/rstb.2015.0065.
11. Moroz, L. L. (2009). On the independent origins of com-
GREY MATTERS JOURNAL AT VASSAR COLLEGE | ISSUE 6 61 REFERENCES
plex brains and neurons. Brain, Behavior and Evolution, 74(3), 177–190. doi:10.1159/000258665.
12. Chung, W. S., Kurniawan, N. D., & Marshall, N. J. (2022). Comparative brain structure and visual processing in octopus from different habitats. Current Biology, 32(1), 97–110. doi:10.1016/j.cub.2021.10.070.
13. Michael J. (2021). What do we mean when we talk about “structure/function” relationships? Advances in Physiology Education, 45(4), 880–885. doi:10.1152/advan.00108.2021.
14. Preti, M. G., Van De Ville, D. (2019). Decoupling of brain function from structure reveals regional behavioral specialization in humans. Nature Communications, 10(1), 4747. doi:10.1038/s41467-019-12765-7
15. von Bartheld, C., Bahney, J., Herculano-Houzel, S. (2016). The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting: Quantification of neurons and glia in human brain. Journal of Comparative Neurology, 524. doi:10.1002/ cne.24040.
16. Kramar, M., & Alim, K. (2021). Encoding memory in tube diameter hierarchy of living flow network. Proceedings of the National Academy of Sciences of the United States of America, 118(10). doi:10.1073/pnas.2007815118.
17. 17. Clark, E. G., Hutchinson, J. R., Darroch, S. A. F., Mongiardino Koch, N., Brady, T. R., Smith, S. A., & Briggs, D. E. G. (2018). Integrating morphology and in vivo skeletal mobility with digital models to infer function in brittle star arms. Journal of Anatomy, 233(6), 696–714. doi:10.1111/joa.12887.
18. Clark, E. G., Kanauchi, D., Kano, T., Aonuma, H., Briggs, D. E., Ishiguro, A. (2019). The function of the ophiuroid nerve ring: How a decentralized nervous system controls coordinated locomotion. Journal of Experimental Biology, 222(2). doi:10.1242/jeb.192104.
19. Carls-Diamante S. (2022). Where is it like to be an octopus? Frontiers in Systems Neuroscience, 16. doi:10.3389/ fnsys.2022.840022.
20. Moreno-Jiménez, E. P., Flor-García, M., Terreros-Roncal, J., Rábano, A., Cafini, F., Pallas-Bazarra, N., Ávila, J., Llorens-Martín, M. (2019) Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nature Medicine, 25, 554–560. doi:10.1038/s41591-0190375-9.
21. Yang, Q., Hong, Y., Zhao, T., Song, H., & Ming, G. L. (2022). What makes organoids good models of human neurogenesis? Frontiers in Neuroscience, 16, 872794. doi:10.3389/fnins.2022.872794.
22. Becker, T., & Becker, C. G. (2022). Regenerative neurogenesis: The integration of developmental, physiological and immune signals. Development, 149(8). doi:10.1242/ dev.199907.
23. Rentzsch, F., Layden, M., & Manuel, M. (2017). The cellu-
lar and molecular basis of cnidarian neurogenesis. Wiley Interdisciplinary Reviews Developmental Biology, 6(1). doi:10.1002/wdev.257.
24. Marlow, H. Q., Srivastava, M., Matus, D. Q., Rokhsar, D., & Martindale, M. Q. (2009). Anatomy and development of the nervous system of Nematostella vectensis, an anthozoan cnidarian. Developmental Neurobiology, 69(4), 235–254. doi:10.1002/dneu.20698.
25. Gage F. H. (2019). Adult neurogenesis in mammals. Science, 364(6443), 827–828. doi:10.1126/science.aav6885.
26. Booker, R., & Truman, J. W. (1987). Postembryonic neurogenesis in the CNS of the tobacco hornworm, Manduca sexta. I. Neuroblast arrays and the fate of their progeny during metamorphosis. Journal of Comparative Neurology, 255(4), 548-559. doi:10.1002/cne.902550407.
27. Eriksson, P., Perfilieva, E., Björk-Eriksson, T., Alborn, A.M., Nordborg, C., Peterson, D. A., & Gage, F. H. (1998). Neurogenesis in the adult human hippocampus. Nature Medicine, 4, 1313–1317. doi:10.1038/3305.
28. von Bernhardi, R., Bernhardi, L. E., & Eugenín, J. (2017). What is neural plasticity? Advances in Experimental Medicine and Biology, 1015, 1–15. doi:10.1007/978-3-31962817-2_1.
29. Du Plessis, A., Broeckhoven, C., Yadroitsava, I., Yadroitsev, I., Hands, C., & Bhate, D. (2019). Beautiful and Functional: A review of biomimetic design in additive manufacturing. Additive Manufacturing, 27. doi:10.1016/j. addma.2019.03.033.
30. Flesher, S. N., Collinger, J. L., Foldes, S. T., Weiss, J. M., Downey, J. E., Tyler-Kabara, E. C., Bensmaia, S. J., Schwartz, A. B., Boninger, M. L., & Gaunt, R. A. (2016). Intracortical microstimulation of human somatosensory cortex. Science Translational Medicine, 8(361). doi:10.1126/scitranslmed.aaf8083.
31. Disouky, A., & Lazarov, O. (2021). Adult hippocampal neurogenesis in Alzheimer’s disease. Progress in Molecular Biology and Translational Science, 177, 137–156. doi:10.1016/bs.pmbts.2020.09.002.
32. Goel, P., Chakrabarti, S., Goel, K., Bhutani, K., Chopra, T., & Bali, S. (2022). Neuronal cell death mechanisms in Alzheimer’s disease: An insight. Frontiers in Molecular Neuroscience, 15, 937133. doi:10.3389/fnmol.2022.937133.
33. Rahman, A. A., Amruta, N., Pinteaux, E., & Bix, G. J. (2021). Neurogenesis after stroke: A therapeutic perspective. Translational Stroke Research, 12(1), 1–14. doi:10.1007/ s12975-020-00841-w.
GREY MATTERS JOURNAL AT VASSAR COLLEGE | ISSUE 6 62 REFERENCES
GREY MATTERS JOURNAL AT VASSAR COLLEGE | ISSUE 6 63 REFERENCES

GREY MATTERS JOURNAL AT VASSAR COLLEGE | ISSUE 6 64 REFERENCES





























 by Daniella Lorman/ art by Ava Sclafani
by Daniella Lorman/ art by Ava Sclafani
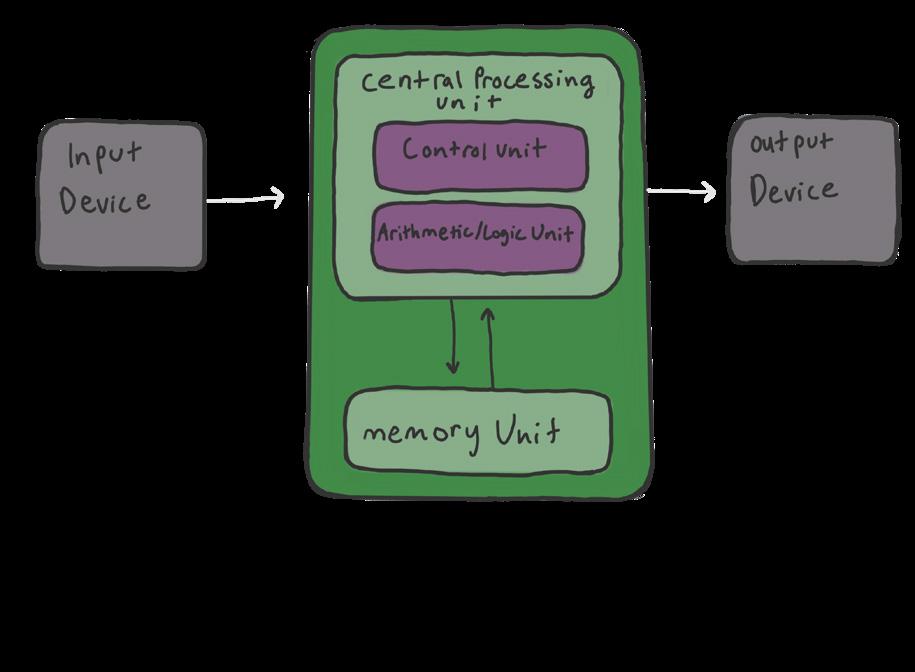

 by Jaclyn Narleski/ art by Arden Spehar
by Jaclyn Narleski/ art by Arden Spehar