GOLD (III) – antibiotic resistance
Novel antibiotic class based on gold(III)complexes (G3Cs) that drastically improves the efficacy, safety, and cost of combatting multidrug-resistant bacteria
antibiotic resistance
wound infections
EU patent granted multitarget activity




Objective: Improve patients health, decrease treatment failure and hospitalization time



TRL : 3 – Applied research - first laboratory tests completed; proof of concept
Gold(III) antibiotic



Development of a new family of irreversible antimalarial drugs (YAT2150) with a novel mechanism of action
malaria new chemical family
new mode of action
Objective: Develop new antimalarial drugs for the post-artemisinin era


TRL : 3 – Applied research - first laboratory tests completed; proof of concept
PLP-3 – antibiotic resistance
Novel cyclic peptide designed and synthesized with antibacterial activity against the most problematic Gram negative pathogens
infectious disease
therapeutics
Objective: Diminish the morbidity, mortality, and health costs associated with MDR and pandrugresistant A. baumannii
TRL : 3 – Applied research - first laboratory tests completed; proof of concept
Point-of-care test to assess the risk of death in all types of infections (in all febrile patients) based on STREM-1 quantification
point-of-care
fever severity triage
clinical decision support medical device

Objective: Improve decision making by clinicians to reduce mortality and optimize referrals due to infections

TRL : 3 – Applied research - first laboratory tests completed; proof of concept


1 4
3 Get in touch: innovation@isglobal.org 2 infectious diseases What we are looking for Environmental health hazards Non-communicable diseases Emerging infections and the growing epidemic of AMR Poverty-related and neglected infectious diseases A research and translation institute in Global Public Health ISGlobal Innovative Biomedical Projects Business consultants Software developers Investors & Funding opportunities Partners & Collaborations Accelerators & Incubators
YAT compound – malaria
B-TRIAGE – infectious diseases
antibiotic resistance
peptide
bactericidal PLP-3
YAT compound
Unmet clinical need
• Antibiotic resistance was responsible for 1.27 M deaths worldwide in 2019 By 2050, 10 M people may die every year from bacterial infections, surpassing the number caused by cancer
• It causes 2.5 M days of extra hospitalization per year throughout the EU resulting in huge costs of 900 M€.
• The global Antibiotics market size is estimated to be $63.2 B by 2027, with a CAGR of 5.1% from 2020 to 2027.

• Among the 45 antibiotics in clinical phases, only six fulfil at least one of the WHO innovation criteria
Intellectual Property
• ISGlobal (50 %) and University of Almeria (50 %) share joint ownership.





• European patent granted (EP3564246A1) in June 2020 with priority date May 2018 Validation completed in 6 EU countries (Germany, Denmark, Belgium, Switzerland, France and Spain) and the UK
• PCT patent application (WO2019211222A1) entering national phases in Canada, US, Brazil, China, India, Japan, South Korea and Mexico
Competitive Advantage
• New small molecule gold (III)-chemical class







• Antibacterial activity against Gram + and GramWHO ESKAPE priority pathogens
• High synergy with currently used antibiotics.
• Lower minimal inhibitory concentrations than conventional antibiotics where resistance is observed.
• Novel mechanism of action
• Service agreement signed with NIAID preclinical services for five years.

Partners
Technology
• Potent Antibacterial The current HIT possesses low MIC values for Pan-R A baumannii (0 06 - 4 μg/ml), ESBLproducing E. coli (4 μg/ml), MRSA (0,03-0,5 μg/ml) and MDR P. aeruginosa (8 μg/ml), including carbapenemresistant isolates.
• No toxic effect in vivo at a dose of 5-8 mg/kg in mice
• Easy access to new derivatives. Highly modular structure that can be modified in several positions.
• High synergy with colistin and amikacin against resistant strains of A baumannii and P aeruginosa
• Chemically stable in the presence of reducing agents of living organisms (glutathione and ascorbic acid) and under physiological conditions
• No resistant mutants were obtained after 30 days of serial passages at different, neither in multiple step experiments nor in one-step experiments.
• Confirming multitarget activity through computational studies

• Currently performing encapsulation assays of the drug into nanoliposomes and assessing their citotoxiciy profile.
• The nanoparticles will be loaded into wound dressings to address wound infections
Development
• LEAD candidate expected in late-2023.
• Lead Optimization to start in 2024, completion expected by mid-2025 with an estimated cost of 1,5-2 M€
• OUR ASK: CO-DEVELOPMENT, LICENSE AGREEMENT and/or INVESTMENT


Our Team

Get in touch: innovation@isglobal.org
Metalloantibiotics against MDR Bacteria
Sara Soto Associate Research Professor ISGlobal
Joan Bigorra Director of Innovation ISGlobal
Oscar Casado Innovation Manager ISGlobal
•
Marina Espriu Head of Technology Transfer ISGlobal
Raquel María González Organic Chemistry University of Oviedo
€1,5-2M
Antibacterial cyclic peptide (PLP-3)






Unmet clinical need

• Antibiotic resistance was responsible for 1.27 M deaths worldwide in 2019 By 2050, 10 M people may die every year from infections caused by multidrug resistant bacteria, becoming the number 1 cause of global deaths.
• It causes 2.5 M days of extra hospitalization per year throughout the EU resulting in huge costs of 900 M€.
• The global Antibiotics market size is estimated to be $63.2 B by 2027, with a CAGR of 5.1% from 2020 to 2027
Intellectual Property
• ISGlobal (47 %), Institute for Research in Biomedicine Barcelona (40 %), Barcelona Clinic Hospital (7 %) and University of Barcelona (6 %) share joint ownership.


• European patent application submitted 27th of May 2022 Positive EESR after EPO examination
• PCT application filled in May 2023 aimed at broadening the protection for the countries of interest.
Competitive Advantage
• Novel cyclic peptide designed and synthesized
• Antibacterial activity against the most problematic Gram-negative and Gram-positive resistant strains, including A. baumannii, P. aeruginosa, K pneumoniae, S Aureus and E faecium
• Low in vitro toxicity in human cells

• Bactericidal and very rapid killer.
• Service agreement signed with NIAID preclinical services for five years
 Ballesté Associate Research Professor ISGlobal-Clínic Hospital
Ballesté Associate Research Professor ISGlobal-Clínic Hospital


• Potent Antibacterial The cyclic peptide possesses low Minimal Inhibitory Concentration (MIC50) values
• Stable and active under physiological conditions. No significant changes in antimicrobial activity of PLP-3 in the presence of human albumin were observed.
• Toxicity in human cells. IC50 values of ca 227 μg/ml for XTT assays indicating up to >100 folds over MIC values for strains of the tested bacterial species over cells.
• IC50 of 48 μg/ml for haemolysis assays
Technology Development
• We are currently completing the Hit-to-Lead stage.
• Presently performing assays to determine ADMET studies and PK screening in mouse.
• In vivo peritonitis sepsis model and antibiofilm activity to be assessed during 2024
• OUR ASK: CO-DEVELOPMENT, LICENSE AGREEMENT and/or INVESTMENT.

Our Team

Get in touch: innovation@isglobal.org
Clara
Oscar Casado Innovation Manager ISGlobal
Jordi Vila Research Professor ISGlobal-Clínic Hospital
Partners
Núria
€1,5-2M Microorganism (resistant strains) MIC50 * MIC90 * Acinetobacter baumannii 1 2 Klebsiella pneumoniae 4 16 Pseudomonas aeruginosa 4 8 Staphylococcus aureus 4 8 Enterococcus faecium 2 2 *in µg/mL
Elisabet Guiral Project Manager ISGlobal
Martín Predoctoral fellow ISGlobal
Unmet clinical need
Novel Anti-infective Targets
PROTEINS AND GENES IDENTIFIED AND VALIDATED AS POTENTIAL TARGETS FOR DEVELOPING NEW ANTIVIRULENCE ANTIMICROBIALS Technology
• Antibiotic resistance was responsible for 1.27 M deaths worldwide in 2019. By 2050, 10 M people may die every year from bacterial infections, surpassing the number caused by cancer.
• It causes 2.5 M days of extra hospitalization per year throughout the EU resulting in huge costs of 900 M€.
• The global Antibiotics market size is estimated to be $63.2 B by 2027, with a CAGR of 5.1% from 2020 to 2027
Intellectual Property
• Potential development of new molecules taking aim to block the identified targets.
• High likelihood of generation of new IP
• Ideally IP ownership is 50:50
Competitive Advantage
• Targets known and validated during 20 years of laboratory work
• Gene-oriented: antivirulence approach has the potential to avoid or minimize resistant development if fewer genes are impacted.
• Identification of the genes that make the bacteria virulent, opens the door to blocking them and having more options for their neutralization.
• Great experienced team of researchers with a high background in microbiological assays
• High capacity of testing at ISGlobal facilities for the antivirulence activity of the new compounds.

• New membrane protein related to haemolysin toxin identified. Bacteria: E coli Diseases involved: UTIs and neonatal sepsis
• Cytotoxic Necrotizing Factor-1 (CNF1) Toxin Bacteria: E coli Diseases involved: UTIs and neonatal sepsis
• FimH/FimA: type 1 fimbriae. Responsible for adherence and biofilm Bacteria: E coli
• P-fimbriae Responsible for adherence to the kidney Bacteria: E coli
• OmpA/OmpF. Triggering biofilm formation and efflux. Bacteria: E coli and other Gram-negative
• Identification of the purL gene as essential for biofilm formation in E. coli. Work published here.
• Ag43 expression enhances biofilm activity. Bacteria: E. coli.
• Outer Membrane Protein C (OmpC) and TolC Responsible of drug efflux Bacteria: E coli
• PilQ (type IV Pili). Responsible of promoting adhesion. Bacteria: E. coli, P. aeruginosa.
• LpxD, related to the biosynthesis of lipid A Bacteria: Gramnegative
Development
• Currently in the phase of screening, aiming to partner with computational chemistry companies to design new candidates







• Looking for CO-DEVELOPMENT and/or RISKSHARING collaborations for rational drug design and HTS.

Our Team

Get in touch: innovation@isglobal.org
Sara Soto Associate Research Professor ISGlobal
Joan Bigorra Director of Innovation ISGlobal
Oscar Casado Innovation Manager ISGlobal
Marina Espriu Head of Technology Transfer ISGlobal
Discovery Preclinical
Compounds for malaria treatment Partners






Unmet clinical need

• Malaria is still a major global health problem According to WHO, in 2021, it caused 619,000 deaths, mainly children under five years old and pregnant women
• The parasite can kill within 24 hours of symptom onset if left untreated
• The main threat to malaria elimination is the evolution by the parasite of resistance to every drug deployed against it.
• New drugs with new MoA, representing a new chemical class and good therapeutic index are urgently needed to overcome resistance issues
Intellectual Property
• ISGlobal (40 %), Institute of Bioengineering of Catalonia (40 %), and University of Barcelona (20 %) share joint ownership.




• PCT application submitted 21st October 2022

• Excellent perspectives to be granted after EESR and ISR positive evaluation reports.
• National phases entry to be expected in April 2024.
Competitive Advantage
•High activity in vitro including chloroquine- and artemisinin-resistant lines
• New chemical family with no other antimalarial drugs described and new mechanism of action.
• Theranostic agent for malaria (it turns fluorescent when entering the parasite) promoting its use in diagnostics tools
• Quick and easy synthesis (2 steps) from commercially available sources


• High stability The compounds have a long shelf life (months) at room temperature.
Technology
• Potent Antimalarial Family of compounds around YAT2150 (HIT compound) possess high in vitro activity and quite a good selectivity index:
• Irresistible antimalarial drug. No resistant P. falciparum strains emerged in vitro after 60 days of cross-resistance assessment in P falciparum lines
• Preliminary data suggests that YAT2150 will be active against all Plasmodium species causing malaria targeting protein aggregation, which is essential for the viability of the parasite.

• YAT2150 has a low teratogenic index (TI) in the zebrafish model (1 15), significantly below that of artemisinin controls (2 04)
Development
• We are currently completing the Hit-to-Lead stage (TRL 3) :
• Presently preparing in vivo experiments in a murine model of malaria and identifying proteins interacting with, or being affected by YAT2150.
• Transmission-blocking potential in mosquitoes to be assessed in the following months
• OUR ASK: CO-DEVELOPMENT, LICENSE AGREEMENT and/or INVESTMENT.

Our Team
Get in touch: innovation@isglobal.org
Diego Muñoz-Torrero Associate Professor of Organic and Medicinal Chemistry Universidad de Barcelona
Oscar Casado Innovation Manager ISGlobal
Xavier FernàndezBusquets Associate Research Professor ISGlobal-IBEC
Claudia Camarero Predoctoral fellow ISGlobal-IBEC
€1,5-2M *CC50: Cytotoxic Concentration Compound IC50 (nM) CC50 (µM)* SI (Selectivity Index) YAT2150 90 3.4 37.8 PRC25 47 4.8 102 EMA377 59 57 956
Lucía Román Predoctoral fellow ISGlobal-IBEC
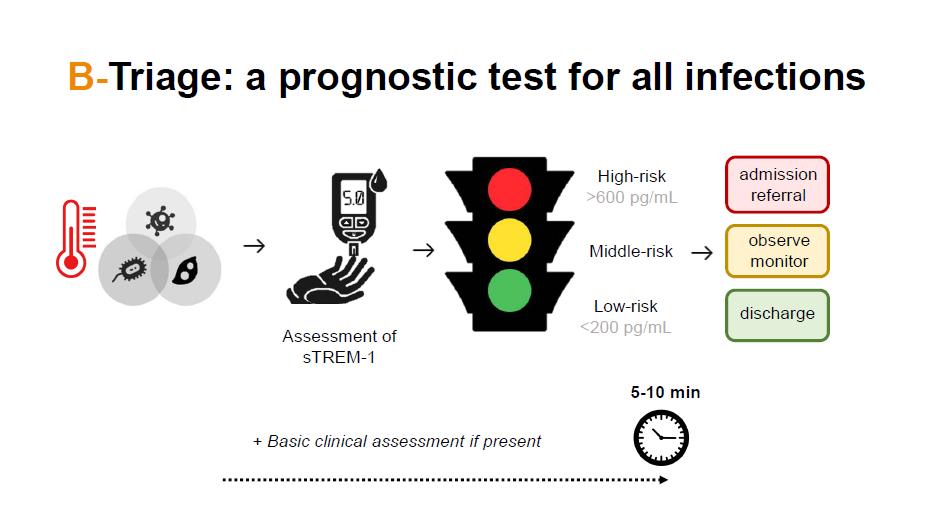
A PoC test for triaging severity in fevers
Partners






• Hospitals & Medical Research Centers in EU, America and Africa.
• Technological and Regulatory partners in EU
Unmet clinical need
• Main clients are Emergency Departments Primary Health Care facilities attending patients
• Total Addressable Market of $1.3 Billion, 17%.


• 17M deaths each year due to infections cause mortality), mostly in children
• Costs associated with severe infections billion per year in the US (∼5 2% of costs)
• Product and services to improve triaging fevers.
Intellectual Property
• Patentability and FTO studies ongoing Detection platform already protected; novel sTREM-1 antibody to be protected
• Class C In Vitro Diagnostic Medical Device (IVD).
• Milestone for CE marking in 2026
Competitive Advantage
• B-Triage is fast, accurate, affordable, portable and stable Needs only a drop of blood to give results
• Based on the most accurate prognostic marker for infections (sTREM-1). Predicts 28day mortality in 1,000 febrile children better than any other marker used in the clinical practice or developed by competitors
Technology Development
1) sTREM-1 recognition by a novel specific antibody
2) the use of a innovative detection system based on magnetic pre-concentrate of analytes to achieve higher sensitivity than other immuno-based assays
3) the system is coupled to an electrochemical readout to give a quantitative result (glucometer-like)
• B-Triage is at TRL 3, currently working on a laboratory prototype.
2024 2025 2026
• Minimal Viable Product (MVP) to be expected in early 2024
• Exploitation strategy includes either spin-off creation or licensing to a well-established IVD company.
• Funding of 8 M€ until 2027 through ECHILIBRIST project funded by HORIZON EUROPE

Our Team
M Isabel Pividori, PhD Professor and Group Leader Biosensor design Lead

 Kevin Kain, MD Director of SAR research Biomarker expert Toronto University
Kevin Kain, MD Director of SAR research Biomarker expert Toronto University



Get in touch: innovation@isglobal.org
UAB
Jofre-Ferrer Dalmau, PhD CEO at BioEclosion Prototyping Lead BioEclosion
Quique Bassat, PhD ICREA Research Professor, Pediatrician Clinical Lead ISGlobal
Bàrbara Baro, PhD Biomedicine, Laboratory and field work Entrepreneurial Lead ISGlobal
2022









































 Ballesté Associate Research Professor ISGlobal-Clínic Hospital
Ballesté Associate Research Professor ISGlobal-Clínic Hospital























 Kevin Kain, MD Director of SAR research Biomarker expert Toronto University
Kevin Kain, MD Director of SAR research Biomarker expert Toronto University


