in on
weigh
renal
denervation as a blood pressure lowering therapy.
denervation
as US FDA considers device approvals

“Advancements like renal denervation are imperative to enhancing shared decision-making that can lead to better treatment and outcomes for patients,” the Society for Cardiovascular Angiography & Interventions (SCAI) said in August amid a regulatory review of two renal denervation systems for reducing blood pressure in adults with uncontrolled hypertension.

SCAI is the latest society, and the first in the USA, to endorse renal denervation as a device-based option for individuals with high blood pressure who may not respond to or tolerate existing treatments including drug therapy or lifestyle modification. In August, the interventional cardiology organisation issued a position statement in which it emphasised the conditions under which interventionalists may opt to select renal denervation for their patients.

“Device therapies targeting the renal sympathetic nervous system hold promise as adjuncts to abate or interventions to abolish hypertension, depending upon the underlying severity of blood pressure elevation,” said Herbert Aronow (Michigan State University College of Human Medicine, East Lansing, USA), chair of the writing group that composed the position paper. “This statement emphasises that, with appropriate patient selection, evaluation, and strict implementation of operator training standards and facility requirements, renal denervation treatment can be provided in an optimal fashion to this patient population.”
In Europe, where the use of renal denervation is more advanced, proponents of the technique now have the added weight of guidance from the European Society of Hypertension (ESH), which for the first time includes the statement that the technique can be proposed as an adjunctive therapy in select patients with resistant hypertension.


The guidelines were first presented during the ESH’s 32nd annual European meeting on hypertension and cardiovascular protection (24–26 June, Milan, Italy). They cite recent randomised trials, including the RADIANCEHTN and RADIANCE II trials, investigating the Paradise (ReCor Medical) ultrasound renal denervation system, and SPYRAL HTN-OFF MED, investigating the Symplicity Spyral (Medtronic) radiofrequency device, as having shown that the treatment can be associated with a significant, albeit not marked, reduction in office and ambulatory blood pressure in patients with uncontrolled hypertension.
An earlier version of the ESH guidelines—which were published jointly with the European Society of Cardiology (ESC) in 2018—had advocated against the routine use of device-based therapies for hypertension, until further evidence of their safety and efficacy came to light. But, with the addition of evidence from the latest generation of sham-controlled trials has come a revitalised optimism in the potential impact of renal
The new guidelines state that the therapy can be considered as an option if patients have an estimated glomerular filtration rate (eGFR) of at least 40ml/min/1.732, in spite of the use of antihypertensive drug combination therapy, or if drug treatment elicits serious side effects and poor quality of life. And, for patients with true hypertension, the document notes that renal denervation can be considered as an additional therapy in patients with true resistant hypertension and an eGFR of at least 40ml/min/1.732
“In the last guidelines, done in 2018 by the European Society of Hypertension and the European Society of Cardiology, it was said that there was no evidence that renal denervation lowered blood pressure, because of the negative data from the first fully controlled trial,” Giuseppe Mancia (University MilanoBicocca, Milan, Italy), who co-chaired the writing committee for guidelines, told Cardiovascular News, discussing the development of the renal denervation field.

Hot Line trials add weight to case for intravascular imaging during PCI


DATA FROM THREE randomised trials and a meta-analysis presented at the European Society of Cardiology (ESC) congress (25–28 August, Amsterdam, The Netherlands) offer new insights into the use of intravascular imaging— either optical coherence tomography (OCT) or intravascular ultrasound (IVUS)—to optimise outcomes during percutaneous coronary intervention (PCI).
“Since then, we have had five or six studies with a shamdenervated group in which there was a reduction in both office and ambulatory blood pressure after renal denervation. In addition, there are data from registries, now up to three years from around 3,000 patients who have undergone renal denervation, showing that the reduction in blood pressure is Continued on page
Attendees to the ESC 2023 main arena session heard evidence from the ILUMIEN IV trial, looking at OCT-guided PCI in high-risk patients; the OCTOBER trial, which assessed OCT-guidance in bifurcation lesions; and OCTIVUS, a comparison of OCT and IVUS in a broad population of patients. The session finished with the presentation of a “real-time” updated network meta-analysis, integrating data from both the ILUMIEN IV and OCTOBER trials.

Presenters and panellists broadly agreed that the new data, taken in totality, make a strong case for the incorporation of intravascular imaging into clinical practice.

First presenting ILUMIEN IV results, investigator Ziad Ali (St Francis Hospital, Roslyn, USA) reported that the use of OCT contributed to a larger minimum stent area than when using angiographic guidance, but the trial found that there was no reduction in the co-primary endpoint of target vessel failure at two years. The impact of the pandemic on enrolment may have played a role in the neutral result, Ali said.
The trial, results of which were published simultaneously to presentation in the New England Journal of Medicine (NEJM), was conducted at 80 sites in 18 countries, randomising a total of 2,487 patients to either OCT-guided PCI (1,233) or
Global Cardiovascular Awards put the spotlight on innovation and excellence in the cardiovascular field
CARDIOVASCULAR DISEASES ARE THE leading cause of death worldwide, responsible for more than 20 million deaths per year, according to latest figures. From the earliest cardiac surgical procedures, to the advent of transcatheter therapies such as transcatheter aortic valve implantation (TAVI) and beyond, human ingenuity, innovation and effort have been key to turning the tide against this indiscriminate killer.
To recognise the enormous amount of work that is being done across the globe to tackle the burden of cardiovascular disease and improve patients’ lives for the better, Cardiovascular News is proud to be a part of the first ever Global Cardiovascular Awards taking place in spring 2024. The Global Cardiovascular Awards is a free-to-enter recognition scheme, seeking to highlight the groundbreaking contributions of individuals and organisations in the pursuit of better cardiovascular care for all.
Submissions for entry are now open until autumn 2023 with categories spanning 16 areas in total. There are awards that touch on the work of clinicians, researchers, healthcare providers, industry, innovators and institutions. All entries will be judged by an expert panel, comprising leaders from the clinical and business fields, and winners will be announced at a ceremony taking place in central London on 14 March 2024.
Judges include Ian Meredith, a clinical and interventional cardiologist from Monash University (Melbourne, Australia) whose extensive career has spanned both clinical and industry roles, most recently as global chief medical officer of Boston Scientific. Ruggero De Paulis (European Hospital UniCamillus University, Rome, Italy), an innovator in cardiac surgery and the chair of the European Association of Cardio-Thoracic Surgery (EACTS) also joins the panel, alongside Chris Landon, the business leader for Image Guided Therapy Devices at global medical device
business Philips, who will bring expertise in cardiac surgery and imaging, respectively. A full judging panel will be announced on the Global Cardiovascular Awards website.
Do you have a colleague who has dedicated their life to shaping the global landscape of cardiovascular care? Perhaps they are a suitable nominee for the Lifetime Contribution to Cardiovascular Care award. Are you working with a young investigator or clinician who has made huge strides early in their career? They could be an ideal candidate for the Future Leader award.
Recognising that efforts to improve multidisciplinary working are fundamental to gaining the best outcomes for patients, the Heart Team of the Year award will identify the team with the greatest commitment to cross-disciplinary working in the patient interest. Other patient-facing awards also include the Best Education or Awareness Campaign, which will recognise the most impactful campaign aimed at facilitating access to treatment, or the Best Prevention Campaign, comprising initiatives dedicated to the prevention of cardiovascular disease.
The Digital Innovation Award pays notice to the increasing use of novel digital, mobile or wearable technologies in cardiovascular care, and will crown the best use of digital tools in this space. Applications including smart use of data analytic techniques or machine learning may also be eligible for inclusion.


Elsewhere, the awards look at the dynamic work being done by industry to drive forward important developments in device technologies and techniques. Across four categories, the Global Cardiovascular Awards will honour major advances in technologies and techniques in areas such as interventional cardiology, cardiac surgery, patient and operator safety, as well as cardiac imaging. Categories including Best Cardiovascular Product Launch and CEO of the Year will further recognise industry excellence.
in brief The latest stories from the vascular world
n STS HARNESSES
BIG DAT FOR RISK
CALCULATOR:
The Society of Thoracic Surgeons (STS) has launched a new digital tool, drawing on data from more than eight million procedures collected through its Adult Cardiac Surgery Database, which is intended to act as a powerful tool for decision-making on surgical risk. STS president Thomas MacGillivray (Washington DC, USA) spoke to Cardiovascular News about this and other changes to the Society’s digital offering.

For more on this story go to page 8.
n TEN-YEAR TAVI DATA:

A decade of follow-up data from the NOTION trial, looking at transcatheter aortic valve implantation (TAVI) versus surgery in low-risk patients are now available. Ten-year data were recently shared at the European Society of Cardiology (ESC) congress (25-28 August, Amsterdam, The Netherlands), where Troels Hojsgaard Jorgensen (Copenhagen, Denmark) reported that patients undergoing the two approaches had similar risk of all-cause mortality, stroke or myocardial infarction.

For more on this story go to page 14.
n COVID-19 AND STEMI:
New research sheds light on the impact of COVID-19 on the care of patients with ST-elevation myocardial infarction (STEMI). This includes the loss of life associated with delayed or missed care during the initial lockdown periods, or the effect of the disease on the coronary arteries.
For more on this story go to page 23.
Editor-in-chief: Simon Redwood | Publisher: Roger Greenhalgh | Content Director: Urmila Kerslake | Head of Global News: Sean Langer
Senior editor: Will Date will@bibamedical.com | Editorial contribution: Jamie Bell, Jocelyn Hudson
Design: Terry Hawes, Wes Mitchell and David Reekie
Advertising: Melanie Stevenson melanie@bibamedical.com
Subscriptions: subscriptions@bibamedical.com







YOU’RE INVITED…

AWARDS 2024


SHERATON GRAND PARK LANE
LONDON, UNITED KINGDOM
The Global Cardiovascular Awards acknowledge and celebrate the inspirational figures shaping the future of cardiovascular care across the world. Join us for a wonderful evening celebrating achievements, fostering innovation and promoting collaboration in the field of cardiovascular medicine!
SAVE THE DATE
GLOBAL CARDIOVASCULAR AWARDS 2024 CATEGORIES
Innovation in Interventional Cardiology
Innovation in Patient and Operator Safety
Best Cardiovascular Product Launch
Contribution to Diversity in Cardiovascular Care
Future Leader Award
CEO of the Year
Best Education Awareness Campaign
Contribution to Sustainability
Innovation in Cardiac Surgery
Innovation in Cardiac Imaging
Digital Innovation Award



Lifetime Contribution to Cardiovascular Care
Heart Team of the Year
Best Prevention Campaign
Collaboration of the Year
Clinical Research Excellence Award
Hot Line trials add weight to case for intravascular imaging during PCI

Continued from page 1
angiography-guided PCI .
Ali reported that the minimum stent area after PCI was 5.72±2.04mm2 in the OCT group and 5.36±1.87mm2 in the angiography group, while target-vessel failure within two years occurred in 88 patients (7.4%) in the OCT group and in 99 patients (8.2%) in the angiography group.
“OCT-guided PCI led to a larger minimum stent area, enhanced the safety of the PCI procedure and resulted in a nearly two-thirds reduction in stent thrombosis during two-year follow-up,” commented Ali of the Abbott-funded trial’s results. “However, in this trial OCT guidance did not reduce the two-year rate of target vessel failure compared with angiography-guided PCI because of a low and nearly identical rate of target vessel revascularisation in the OCT-guided and angiography-guided PCI arms.”
If the results of ILUMIEN IV painted a mixed picture for OCT, the following trial, OCTOBER, appeared to give a firmer answer of its benefit. Ali, Lene Nyhus Andreasen (Aarhus University Hospital, Aarhus, Denmark) delivered findings of the trial in which investigators examined the use of OCT in patients with complex bifurcation lesions.
OCTOBER included 1,201 patients from 38 European centres, and randomised 600 to OCT-guided PCI and 601 to angiography-guided PCI. Those undergoing OCT-guided PCI were treated according to a protocol including a stepwise evaluation of lesion preparation, lesion length, reference sizes, lesion coverage, stent expansion, malapposition, wire positions and ostial results. In the angiography-guided arm, the use of IVUS was allowed in cases requiring treatment of left main artery stenosis.
Reporting the results at two years, which were also published in NEJM, Nyhus Andreasen detailed that the primary endpoint, major adverse cardiac events (MACE), defined as a composite of cardiac death, target lesion myocardial infarction, and ischaemiadriven target lesion revascularisation, occurred in 10.1% of patients in the OCT-guided arm, and 14.1% of patients who underwent angiography-guided PCI. There were no apparent differences in procedural safety, but the volume of contrast and the procedure time were both increased with OCT-guided PCI compared with angiography-guided PCI.
“The OCTOBER trial demonstrated that in patients with complex bifurcation lesions, OCT-guided PCI was associated with better outcomes after two
COVER STORY continued
Societies weigh in on renal denervation as US FDA considers device approvals
Continued from page 1
persistent and also that it is a relatively safe procedure.”
Both the Medtronic and ReCor Medical devices carry the CE mark
years than angiography-guided PCI,” said Nyhus Andreasen. “Procedures using OCT guidance were safe but took longer and the investigators used more contrast. In the angiography-guided arm, IVUS was also used in approximately one in five cases, which reflects current clinical practice for complex PCI procedures in many centres. The results suggest that routine use of structured OCT guidance during PCI of complex bifurcation lesions should be considered to improve prognosis.”
Next came the results of the OCTIVUS trial, an investigator-
OCT group as compared with the IVUS group. At one year after randomisation, the primary endpoint had occurred in 25 of 1,005 patients (2.5%) in the OCT-guided PCI group and in 31 of 1,003 patients (3.1%) in the IVUS-guided PCI group, investigator Duk-Woo Park (Asan Medical Center, Seoul, Republic of Korea) reported.
“Among patients undergoing PCI for diverse coronary artery lesions, OCT-guided PCI was non-inferior to IVUS-guided PCI with respect to a composite of death from cardiac causes, target vessel myocardial infarction, or ischaemia-driven target vessel revascularisation at 12 months after the index procedure,” said Park. “The primary results of OCTIVUS add compelling evidence on the relative efficacy and safety of an OCT-guided strategy compared with an IVUS-guided strategy for PCI.”
Rounding off the session, Gregg W. Stone (Icahn School of Medicine at Mount Sinai, New York, USA) presented the findings of a real-time network metaanalysis, integrating results from both ILUMIEN IV and OCTOBER alongside prior data, to examine the effects of intravascular imaging versus angiographic guidance. The analysis compared the overall effects of imaging in improving outcomes of PCI versus angiography, as well as comparing IVUS and OCT individually to angiography and one another.
The analysis incorporated 20 randomised trials of intravascular imaging-guided PCI compared with angiography-guided PCI in 12,428 patients with chronic and acute coronary syndromes. Of those, 7,038 were randomly allocated to intravascular imaging guidance (including 3,120 patients randomised to IVUS guidance, 2,826 patients randomised to OCT guidance, and 1,092 patients randomised to IVUS or OCT guidance), and 5,390 patients were randomly allocated to angiography guidance. Patients were followed for between six months and five years.The primary endpoint was target lesion failure, defined as a composite of cardiac death, target vessel myocardial infarction, or target lesion revascularisation.
initiated, prospective, multicentre, randomised, openlabel trial conducted at nine sites in South Korea, in which trialists conducted a head-to-head comparison of outcomes for OCT- and IVUS-guided PCI in patients with a broad range of coronary artery lesions. A total of 2,008 patients were randomised in a 1:1 ratio to undergo either OCT-guided or IVUSguided PCI after diagnostic coronary angiography. The primary endpoint was a composite of death from cardiac causes, target vessel myocardial infarction or ischaemia-driven target vessel revascularisation at one year, which was powered for non-inferiority of the
for use in Europe, but neither device is currently approved for commercial use in the USA. This may yet change, with both having filed premarket approval applications with the US Food and Drug Administration (FDA), which are expected to conclude later this year.
Ahead of a likely decision on both applications, the FDA’s Circulatory Systems Devices Panel met in late August to review evidence underpinning the use of the two systems, with voting suggesting that the panel agreed that available data support the efficacy and safety of both of the devices, though voting differed on whether the benefits outweighed the risks for the two devices.
Stone reported that intravascular guidance of PCI resulted in reductions in the primary composite outcome of target lesion failure by 31% compared with angiography guidance of PCI. Stone also noted that there were statistically significant reductions in all-cause death, all myocardial infarction and target vessel revascularisation with intravascular imaging guidance of PCI. The outcomes were similar for OCTguided PCI and IVUS-guided PCI when compared individually against angiography and when compared to each other.

“The results of this network meta-analysis emphasise the importance of physicians using intravascular imaging with either OCT or IVUS to optimise stent outcomes and improve the long-term prognosis of their patients,” Stone said.
For the Paradise system, the committee voted unanimously in support of safety, eight to three in support of effectiveness, with one member abstaining, and 10 voted that the benefits outweigh risks while two disagreed.
For the Symplicity Spyral device, meanwhile, the committee voted unanimously in support of safety, seven in support and six against on effectiveness, while six voted yes, seven against (with tiebreaker by chair) and one abstained on the benefits outweighing the risks.
Following the conclusion of voting, SCAI described the review as a “step forward to advancing access
to additional therapies for people with uncontrolled hypertension” and welcomed the scrutiny of the data.
“It is important we continue to seek out new treatment options for patients, including the use of renal denervation, given the growing global prevalence of uncontrolled hypertension increase year over year,” George Dangas (Icahn School of Medicine at Mount Sinai, New York, USA), SCAI president, said. “The panels’ votes will allow physicians and patients access to a renal denervation procedure for the first time with the potential to improve the treatment and quality of life for those suffering from hypertension.”
“The results of this network metaanalysis emphasise the importance of physicians using intravascular imaging with either OCT or IVUS to optimise stent outcomes and improve the long-term prognosis of their patients.”Lene Nyhus Andreasen presents at ESC 2023. Inset: OCT guidance.
EACTS puts innovation to the fore
Innovation is a part of the DNA of cardiothoracic surgery, Friedhelm Beyersdorf (Heart Center Freiburg University, Freiburg, Germany), immediate past president of the European Association for Cardio-Thoracic Surgery (EACTS) tells Cardiovascular News, discussing the organisation’s commitment to place a renewed focus on innovation within the field.
“SEVENTY YEARS AGO THE
first operation with a heart-lung machine took place,” he comments, noting that subsequent years have heralded major advances in the treatment of paediatric heart disease, valvular, coronary and aortic diseases, as well as in areas such as transplantation, which have transformed outcomes for patients.
Beyersdorf placed the commitment to innovation at the heart of his 2021/2022 presidency of EACTS, a major action point of which was the establishment of the Innovation Summit, a first-of-its-kind event taking place in Paris between 20–22 April, at which experts from the cardiothoracic surgery field, as well as other scientific specialties, share new science and thinking on technologies and techniques that could drive better outcomes for patients with heart and lung disease.
“With huge innovations in other fields of science, this will help us to treat our patients even better,” says Beyersdorf of the aim to learn from ideas outside of the field of cardiac surgery. “There is a saying that the biggest room in the world is the room for improvement, and that is the reason why I said that right now is the perfect time for huge innovations.” In particular, Beyersdorf points to advances in artificial intelligence (AI), augmented reality, molecular medicine, and quantum physics as areas where there is scope for learning and new ideas.
Innovation Summit
The two-day summit was billed as an interactive working event, including 36 presentations delivered to an audience of surgeons, engineers, scientists, cardiologists and industry leaders, with a core focus on developing new ideas and concepts to bring into everyday clinical practice. “These huge innovations, disruptive science, occurs at the border of different scientific fields,” says Beyersdorf. “Very often, everybody thinks in a certain box in their own profession. If somebody else is coming from the outside, that is the advantage of diversity. Somebody else sees it completely differently, and there is not a right or wrong, there is just different.”
Four presentations from the session
have been selected to feature at the Association’s annual meeting in October (4–7 October, Vienna, Austria) with an aim to disseminate new ideas to the wider cardiothoracic surgery community. These will offer updates on topics including opto-electronic implants, an arrhythmia treatment that takes place at a cellular level using small-scale implanted lightemitting diodes (LEDs); mitochondrial transplantation, which sees healthy autologous mitochondria transferred into damaged cardiac cells; myocardial regeneration; and automated reperfusion of the whole body.
The first summit had a wide brief, Beyersdorf explains, but one of the outcomes of the meeting has been to home in on a series of topics in which it is felt that there is a clear case for further innovation (see Five key innovation trends to watch in cardiac surgery).
Though the Innovation Summit is a first-of-its-kind event, the EACTS annual meeting has for a number of years featured on its programme a TechnoCollege component which is aimed at highlighting innovation in technologies
and techniques for cardiovascular and thoracic surgery. The 2022 edition showcased new techniques for aortic valve replacement, robotic technology, and a live case showcasing an endoscopic valve replacement. Each year a panel selects one innovation to be the recipient of its innovation prize, which in 2022 was collected by the developers of a cannula for minimally-invasive central aortic perfusion—MIC-Cannula.
Presently, says Beyersdorf, among the biggest hurdles to innovation is the regulatory landscape. Nowhere is this more evident than in Europe, where the introduction of the EU’s Medical Devices Regulation (MDR) has created new challenges for innovators looking to bring forward new solutions.
Despite these challenges, Beyersdorf says he is confident that through embracing continuing innovation the role of the cardiac surgeon will be scarcely recognisable in decades to come compared to today. “When we look back from 2023 to the 1960s we see a big change. In 20 years I am sure we will look back to 2023 and see something similar.”
Five key innovation trends to watch in cardiac surgery
Extracorporeal circulation
Extracorporeal circulation was first used in 1953, when the first operation with cardiopulmonary bypass (heart-lung machine) was successfully performed. In the following decades miniaturised perfusion systems were developed for lung (extracorporeal membrane oxygenation, VV-ECMO) or heart/ lung replacement (extracorporeal life support, [ECLS], AV-ECMO). Then, just a decade ago, longterm perfusion for healthy organs was made possible (ex vivo perfusion) for organ preservation for transplantation. Most recently, single human organ repair (e.g., for lung transplants) and even multi-organ repair is possible for improved survival results after cardiac arrest (controlled automated reperfusion of the whole body, CARL). “The heartlung machine is a fantastic tool to operate within the heart,” says Beyersdorf. “Now from the groin you can put in some cannulas and repair the body after cardiac arrest.”
Robotics and automation
Robotic cardiac surgery utilises small incisions avoiding the need for a full sternotomy, and has most commonly been used to perform mitral valve surgery
and coronary artery bypass graft (CABG) procedures. “Robotics is really exciting, but it is probably the wrong term,” comments Beyersdorf. “In surgery the robots do not do anything by themselves,” he explains, noting that current robotically-assisted procedures are guided by skilled surgeons. However, the field is advancing, and Beyersdorf points to the recent world-first laparoscopic surgery on the soft tissue of a pig, performed without human guidance. “This will have huge implications for clinical surgery.”
Heart valves for the future
“The Holy Grail is that one day you take a skin cell and grow your new heart valve, your new coronary artery, or even the whole heart out of that,” says Beyersdorf. While he acknowledges that this concept is still “light years” from a new heart being developed using cells harvested from elsewhere in the body, that goal is “coming closer and closer” in the heart valve space.
Artificial intelligence (AI) and augmented reality
In applications such as procedural planning, training and risk prediction, the use of AI and
augmented reality technologies has already begun to enter the cardiac surgery field. “We have teaching tools with augmented reality to the young surgeons. We have 3D [three-dimensional] printing of the heart of the disease patients, especially in congenital cardiac surgery,” comments Beyersdorf.

Improvements in perioperative care

“Surgical techniques themselves are fantastic,” comments Beyersdorf, “and in most instances they are better than interventional techniques”. He notes that for many, the advantage of opting for an interventional procedure is the shorter associated recovery time. However, efforts are being made to ensure that patients undergoing surgery can also expect to benefit from a shorter hospital stay and recovery time.
“We are now on the edge of improving these perioperative techniques in revolutionary ways so that even after huge procedures you might be able to leave the hospital, if not the next day, then on the third or fourth day, and you will experience significantly fewer comorbidities. This will, of course, be great for the patients.”
When we look back from 2023 to the 1960s we see a big change. In 20 years I am sure we will look back to 2023 and see something similar.”
Friedhelm Beyersdorf
STS risk calculator uses power of big data to predict cardiac surgery risk
A new digital tool launched by the Society of Thoracic Surgeons (STS) draws together data from more than eight million procedures collected through the Society’s Adult Cardiac Surgery Database to power decision-making on surgical risk.
According to STS president Thomas MacGillivray (MedStar Heart and Vascular Institute, Washington DC, USA), the Society’s new Operative Risk Calculator, launched in July, will provide a vital tool in counselling patients on their potential procedural risk, as well as making it easier for surgeons to assess risk at the bedside.


“The previous calculator that we had was very good, but it was a little bit clunky,” MacGillivray tells Cardiovascular News. Through the previous generation, users would need access to a desktop computer and were required to fill out several pages of information. The newgeneration risk calculator has all of the information contained on one page and is compatible with mobile devices, making it more user-friendly, according to the STS president.
The risk calculations are based on the most current nationwide data from the STS Adult Cardiac Surgery Database and these are informed by robust risk models that continuously update every
three months, down from every few years under the previous model.
MacGillivray comments that having access to a more up-to-date pool of data will mean that users can be more adaptive based upon evolving trends in the treatment of patients with heart disease.
“The practice of cardiac surgery is changing. With transcatheter therapies, there is less [surgical] aortic valve replacement. Patients who get an aortic valve replacement are different now because most of the patients get a transcatheter valve replacement,” he explains. “With the data and risk models being updated more frequently, you get a more accurate set of data compared to if you had to wait for that every few years.”
Harnessing data will have a positive impact on the way that surgeons are able to communicate surgical risk to their patients, MacGillivray adds, commenting that this is among the benefits of drawing upon real-world information.
“I know from my own practice that if somebody
ESC and EACTS conclude review of left main revascularisation guideline recommendations
A joint review of evidence into the treatment of patients at low surgical risk with left main coronary disease has concluded that both coronary artery bypass graft (CABG) surgery and percutaneous coronary intervention (PCI) are clinically reasonable based on patient preference, available expertise and operator volume.
THIS WAS THE OUTCOME OF A collaborative review undertaken by the European Society of Cardiology (ESC) and the European Association for Cardiothoracic Surgery (EACTS), which included the joint guidelines issued by the two organisations on myocardial revascularisation in 2018. EACTS withdrew its support for the 2018 guidelines, after a BBC investigation cast doubt on the findings of the EXCEL clinical trial, which was among the evidence used to inform the drafting of the guidelines.
The review was authored by a panel chaired by Robert Byrne (RSCI University of Health Sciences, Dublin, Ireland) and Stephen Fremes (Sunnybrook Research Institute, Toronto, Canada), and its outcomes have been published in both the
European Heart Journal and the European Journal of CardioThoracic Surgery
The panel have advised that in future guidelines the class of recommendation and level of evidence for CABG should be class I and level of evidence A, and class IIa and level of evidence A for PCI.
Their report and associated materials are now being considered by the Task Force which is working on a new guideline for chronic coronary syndromes, scheduled for publication in August 2024.
Until then, the ESC and EACTS have stated that local heart teams should consider both the 2018 guidelines and the findings of the expert group when discussing the management of patients with stable coronary artery disease.
comes in to talk about surgery they are very nervous, and their imagination has run wild. So when you do these risk calculators and you show them what their risk is they are quite often relieved and surprised that the risk is as low as it is.
“That is probably one of the greatest benefits of it, that you can both inform the patient with some objective information, and you can reassure the patient and their family with that information and knowing that it is accurate.”
Updates to the risk calculator are the culmination of a long-term project initiated by the STS, which has taken on board the needs of members who use the tool and the database. The organisation has also taken over the management of its database in-house, where previously it was hosted by the Duke Clinical Research Institute.
“In the past we were somewhat dependent on other organisations to be the keepers of our data and to run the data model; we were on their timeline. With the changes, we are now in control of that and we can set the pace, and that is what has allowed us to facilitate these changes,” says MacGillivray.
Further work is being undertaken to enhance the STS National Database, with several changes expected to roll out in the coming months. Specific projects will include risk models for adult congenital heart disease, patients undergoing multiple procedures, and aortic surgery.
Clinical trial to compare PCI and CABG in women and minorities
Researchers will carry out the first clinical trial focusing on women and minority populations to determine which coronary revascularisation procedure best improves their survival and quality of life.
THIS TRIAL WILL BE FUNDED through US$29.9 million from the Patient-Centered Outcomes Research Institute Award to the Icahn School of Medicine at Mount Sinai and Weill Cornell Medicine (both New York, USA).
Icahn Mount Sinai and Weill Cornell Medicine will share the funding, and Maurio Gaudino (Weill Cornell Medicine, New York, USA) will serve as co-principal investigator with Gregg W. Stone (Icahn School of Medicine at Mount Sinai, New York, USA). The award will be distributed in several phases to conduct the two integrated “Revascularization choices among under represented
groups evaluation (RECHARGE) trials called RECHARGE:Women and RECHARGE:Minorities. These are set to launch in October 2023.
They will enrol a total of approximately 1,200 patients across 45 or more sites in the USA and Canada who are eligible for treatment with either PCI or CABG. The overall study will be conducted over a 6.5year period.
“These findings could transform cardiac care for women, Black, and Hispanic patients. If outcomes are better with CABG than PCI in one or both groups then the majority of such patients should undergo surgery. Conversely, if survival and quality of life are similar or better after PCI, this less-invasive approach would be warranted for most patients,” Stone adds.
The Patient-Centered Outcomes Research Institute is an independent, non-profit organisation authorised by the US Congress in 2010. Its mission is to fund research that will provide patients, their caregivers, and clinicians with the evidence-based information needed to make betterinformed healthcare decisions.
This funding award has been approved pending completion of a business and programmatic review by Patient-Centered Outcomes Research Institute staff and issuance of a formal award contract.
The practice of cardiac surgery is changing.”
AI model may improve detection of atrial septal defect
An artificial intelligence (AI) model may be more efficient at detecting signatures of atrial septal defect (ASD) in electrocardiograms (ECG) than traditional methods.
THIS IS ACCORDING TO
investigators from Brigham and Women’s Hospital (Boston, USA) and Keio University (Tokyo, Japan), who have developed a deep learning AI model to screen ECG for signs of ASD.
“If we can deploy our model on a population-level ECG screening, we would be able to pick up many more of these patients before they have irreversible damage,” says Shinichi Goto (Brigham and Women’s Hospital, Boston, USA), corresponding author on the paper published in EClinicalMedicine
ASD is a common adult congenital heart disease caused by a hole in the heart’s septum that lets blood flow between the left and right atria. The symptoms of ASD are typically very mild or, in many cases, non-existent until later in life. Symptoms include an inability to do strenuous exercise, affect the rate or rhythm of the heartbeat, heart palpitations, and an increased risk of pneumonia.
Even if ASD is asymptomatic, it can increase the risk of atrial fibrillation (AF), stroke, heart failure, and pulmonary hypertension. If found early, ASD can be corrected with minimally invasive surgery to improve life expectancy and reduce complications. ASD can be detected in several ways, the largest defects can be found by listening to the heart with a stethoscope, use of an echocardiogram, or screening via ECG.
To see if an AI model could better detect ASD from ECG readouts, the study team fed a deep learning model ECG data from 80,947 patients over 18 who underwent both ECG and echocardiogram to detect ASD. A total of 857 patients were diagnosed with ASD.
The data were collected from three hospitals: two large teaching institutions—Brigham and Women’s Hospital and Keio University, and Dokkyo Medical University (Mibu, Japan), a community hospital. The
Researchers hope to use AI to “revolutionise” prediction of sudden cardiac death
A novel artificial intelligence (AI) model correctly identified patients at near-term risk of sustained ventricular tachycardia (VT) who could potentially benefit from preemptive interventions to prevent sudden cardiac death (SCD).
THE AI MODEL UTILISES A SINGLE-LEAD electrocardiogram (ECG) screening tool that could offer physicians a new approach to SCD risk management, researchers have said, presenting the findings during a late-breaking clinical science session at the 2023 Heart Rhythm Society annual meeting (19–21 May, New Orleans, USA).
As traditional mechanisms for predicting and preventing mid- and long-term SCD are limited, the study sought to understand if AI could be leveraged to better identify near-term occurrences of VT using data from Holter ECG recordings.
The authors of this study developed a deep learning-based model using the first 24 hours of extended Holter monitor recordings, a type of portable electrocardiogram, to predict the risk of sustained (≥30 seconds) ventricular tachycardia (VT) over two weeks.
The model used 78,294 unselected Holter recordings
model was then tested using scans from Dokkyo, which has a more general population and is not specifically screening patients for ASD. The model was more sensitive than using known abnormalities found on ECGs to screen for ASD. The model correctly detected ASD 93.7% of the time, while using known abnormalities found ASD 80.6% of the time.
“It picked up much more than what an expert does using known abnormalities to identify cases of ASD,” Goto says.
One limitation of the study is that the model was trained used samples from academic institutions, which deal more
with rare diseases like ASD. All the patients used to train the model were being screened for ASD and received an echocardiogram, so it is not clear how well the model would work on a general population, which is why they tested it in Dokkyo.
“The model’s performance was retained even in the community hospital’s general population, which suggests that the model generalises well,” Goto adds.
The authors also note that even the use of ECG to detect ASD will not find every defect. Some could slip through both the regular screening and the AI model, though these smaller defects are less likely to require surgical closure.
“The problem of machine learning is that it is a black box—we do not really know what features it picked up,” Goto says.

Results suggest that the technology could be used in population-level screening to detect ASD before it leads to irreversible heart damage, the researchers claim. ECG is relatively low cost and currently performed in many contexts. “Perhaps this screening could be integrated into an annual primary care provider appointment or used to screen ECGs taken for other reasons,” Goto adds.
collected across the USA, UK, France, Czech Republic, South Africa and India. Among 59,302 recordings used for validation, the mean age of patients were 61.3±17.3 years and 40% were male. A total of 222 recordings presented sustained VT with a mean rate of 157±38bpm, and median duration 62 seconds [IQR 42, 173]), with the vast majority (98%) being monomorphic.
“Current methods for predicting SCD are extremely limited. By leaning on artificial intelligence, we hope to revolutionise the way physicians monitor, prevent, and predict SCD, improving the lives of patients while generating cost savings for our healthcare system,” said Laurent Fiorina (Institut Cardiovasculaire Paris Sud, Ramsay, France). “For high-risk patients who suffer from multiple conditions including hypertension, obesity, older age and diabetes, this technology could be lifesaving to help more accurately predict sustained VT and offering physicians important insights to offer early SCD prevention interventions.”
On the internal validation dataset, the model achieved an area under the receiver operating characteristic (ROC) curve (AUC) of 0.939 with a sensitivity of 83.3% and a specificity of 88.7%. On the external validation dataset, the AUC was 0.911 with a sensitivity and specificity of 78.9% and 81.4%, respectively. The AI model correctly predicted VT occurrence in 88% of patients with rapid VT (≥180 bpm). Lastly, the reference model revealed an internal validation AUC of 0.833.
The authors are currently looking to validate the model in future prospective clinical studies. They would also like to extend near-term prevention through ECG monitoring to hospital monitoring or wearable sensors with potential applicability to
US FDA approval for AI TAVI planning software
AN ARTIFICIAL INTELLIGENCE(AI) model for use in pre-procedural planning ahead of transcatheter aortic valve implantation (TAVI)—Precision TAVI (Dasi Simulations)—has been granted approval by the US Food and Drug Administration (FDA).
The technology uses proprietary algorithms to help physicians understand the implications of various treatment options by predicting and visualising the interaction between various devices and the patientʼs unique anatomy. For TAVI procedures, this includes how different prosthetic valves would fit with specific anatomies and valve implantation locations.
“Our game-changing approach has paved the way for the first approved personalised computer simulations in the heart valve space,” Teri Sirset, co-founder and CEO of Dasi Simulations, said in a press release issued by the company.
The technology was initially licensed through The Ohio State University (Columbus, USA) and subsequently through an inter-institutional agreement including Georgia Institute of Technology (Atlanta, USA), Emory University (Atlanta, USA) and Piedmont Hospital Atlanta (Atlanta, USA).
It picked up much more than what an expert does using known abnormalities to identify cases of atrial septal defect.”
European survey highlights lack of uniformity in heart team implementation
A survey of cardiac surgeons and interventional cardiologists from 26 European nations has highlighted “marked variability” in the infrastructure and composition of heart teams in different institutions.
Researchers behind the survey, results of which were published in the journal Interdisciplinary Cardiovascular and Thoracic Surgery, have stated that the findings underscore the need for standardisation on the definitions and guidance for the implementation of heart teams at the institutional level.
The heart team concept—a collaborative approach to determine treatment strategies and to ensure multidisciplinary participation in procedures—has emerged in recent years as an important principal endorsed by a number of professional societies, and is endorsed in US and European guidelines as a requirement for the management of cardiovascular disease
Research from a single centre in Canada has suggested that consultation by a heart team may contribute to an observed benefit in patient outcomes, though further research has suggested that centres with experienced transcatheter aortic valve implantation (TAVI) programmes saw a drop-off in shared decision-making. In a paper published in JAMA: Network Open in 2020, researchers found differing recommendations in up to one-third of cases when comparing the decisionmaking of individual interventional cardiologists to those of a heart team
The purpose of the survey is to determine whether the heart team approach is being applied in different institutions across Europe and to determine the real-life practices of patient management in each institution.

Researchers identified cardiologists and cardiac surgeons through member databases on cardiology and surgical societies, who were contacted with a list of 47 questions focusing on the composition of the heart team in different institutions, execution of the heart team, institutional guidelines for ad hoc interventions, documentation of decisions and understanding of the decision-making process undertaken by the team.
In total 2,188 clinicians were invited to take part in the survey, with 220 ultimately participating. Of the respondents, 64% were cardiac surgeons, and 36% cardiologists.
Of those responding, 91% replied that they did have a heart team within their hospital, with over 50% reporting that such a team should comprise a
cardiac surgeon, general cardiologist, interventional cardiologist, imaging cardiologist and/or anaesthetist.
Furthermore 54% responded that there should be a minimum quorum required for a heart team meeting to take place, with over 50% agreeing that at least a general cardiologist, imaging cardiologist, interventional
team meeting typically lasts for one hour, and 73% noting that this takes place in person.
When asked to provide details of the types of patients discussed during heart team meetings, 82% responded that most patients undergoing percutaneous coronary intervention (PCI) were not discussed, while 56% considered 10–20% of the patients undergoing PCI as being relevant for discussion. Almost half, 49%, of those responding reported a lack of institutional guidelines for ad hoc PCI or other ad hoc interventions.
On decision-making and auditing processes, 83% of respondents reported that the decision-making process of the heart team was guideline-directed, with a combination of factors influencing treatment modalities including risk score, comorbidities, clinical expertise and patient choice.
University Medical Centre, Maastricht, The Netherlands) commented that the need for joint decision-making should be seen as benefitting patients, if shared expertise and decision-making leads to better outcomes.
“This is more important in our field, because interventional cardiology and cardiac surgery have become competing disciplines, fishing in the same river for the same fish,” he commented. “So, it is also important to recognise with an ageing, comorbid population and with patients having disease for life, they need lifetime management.”
cardiologist, cardiac surgeon or heart team coordinator should be present during the heart team meeting.

On the conduct of the heart team, 55% of respondents noted that they had weekly heart team meetings, with 66% of respondents stating that the heart
Heart team survey in numbers
“With increasing patient complexities, advances and emergence of new therapies for cardiac pathologies, the importance of dedicated heart teams cannot be overstated,” authors Umar Imram Hamid (Maastricht University Medical Centre, Maastricht, The Netherlands) et al write in their Interdisciplinary Cardiovascular and Thoracic Surgery paper. “The rationale behind the dedicated heart team is to bring clinicians to the table who are experts in the same pathology but with different skillsets. The survey highlighted the presence of dedicated heart teams for different cardiac pathologies. This allows a more patient-centred approach and tailors the therapy to the requirement of the patient.”
Speaking to Cardiovascular News, the paper’s corresponding author, Peyman Sardari Nia (Maastricht
91% have a dedicated heart team
55% report weekly heart team meetings
82% PCI patients not discussed by heart team
Sardari Nia, who is among the individuals behind the Heart Team Academy, an organisation set up in 2020 to promote better cross-specialty working between the surgical and interventional specialties, commented that he saw a lack of “ownership” among healthcare providers and a culture that has highlighted the talents of “star” individuals over team working within the healthcare system as barriers to change.
The Heart Team Academy, he says, seeks to bring an academic approach to promoting the heart team concept, and aims to bring forward a standardised definition of heart team composition to help centres implementing their own programmes.
Sardarai Nia has previously authored research looking at the benefit of a multidisciplinary approach for the treatment of mitral valve disease, published in the European Journal of Cardio-Thoracic Surgery in 2021, which found that patients had a greater probability of survival at five years if they were treated by a heart team with specific expertise relating to mitral valve pathology.
With increasing patient complexities, advances and emergence of new therapies for cardiac pathologies, the importance of dedicated heart teams cannot be overstated.”










Structural Heart Interventions
Tricuspid repair with TriClip system yields TR reduction and quality-of-life improvement in real-world study
A real-world study of more than 500 patients undergoing transcatheter edgeto-edge repair (TEER) using the TriClip (Abbott) system shows that use of the transcatheter device resulted in a reduction of tricuspid regurgitation (TR) grade to moderate or less for more than three quarters of patients.
THIS WAS AMONG THE SHORT-TERM outcomes of the bRIGHT post-approval study (PAS), presented at EuroPCR 2023 (16–19 May, Paris, France) by Philipp Lurz (University of Leipzig, Leipzig, Germany) and published simultaneously in the Journal of the American College of Cardiology (JACC) bRIGHT PAS, a prospective, single-arm, multicentre registry, including data from 26 sites throughout Europe, with investigators enrolling 511 symptomatic patients with severe TR despite medical therapy and a high risk for tricuspid valve surgery. TR was functional in 90% of subjects, and baseline TR severity in most patients was massive and torrential, at 61.3% and 26.7%, respectively. According to the investigators, patients enrolled in the study had significant
comorbidities, including hypertension (87%), atrial fibrillation (86%), chronic renal disease (40%), diabetes (22%), and prior myocardial infarction (10%).
Study investigators assessed a primary endpoint of acute procedural success, defined as survival to discharge with successful implantation of the TriClip device with a resulting reduction of at least one grade, as well as a secondary endpoint of all-cause mortality or tricuspid valve reintervention or reoperation at one year.
Reporting the study’s results, Lurz and the bRIGHT PAS investigators detail in their JACC paper that the device was successfully implanted in 99% of subjects, with resulting TR reductions to moderate or less at 30 days in 77% of individuals.
The study team also reported improvements in both clinical and quality-of-life metrics, with 79% of participants achieving New York Heart Association (NYHA) functional class I/II, representing a significant reduction compared to baseline, at which point 80% of patients were in NYHA functional class III or IV. Additionally, the investigators found that 56% of patients reported a 15-point improvement in Kansas City Cardiomyopathy Questionnaire (KCCQ) score, a self-assessment of symptoms and quality of life.
In terms of safety, the study’s results show that 2.5% of patients who received the device experienced a major adverse event, a composite of cardiovascular death, heart attack, stroke, new onset of kidney failure and surgery for device-related adverse events.
“Many patients currently undergoing tricuspid TEER are at an advanced stage of the TR disease and experience severe symptoms, impacting their overall
TVT registry analysis finds close to 90% success in mitral TEER procedures
A large registry study of patients undergoing transcatheter edgeto-edge repair (TEER) for degenerative mitral regurgitation (MR) using the MitraClip (Abbott) system, has concluded that the procedure resulted in successful repair in almost 90% of patients.
RESULTS OF THE STUDY,
authored by Raj Makkar (Cedars-Sinai, Los Angeles, USA) and colleagues, have been published in the Journal of the American Medical Association (JAMA), and represent the largest study to date that examines the outcomes for patients treated outside of a clinical trial with transcatheter mitral valve repair, according to researchers.

“Treatment was successful in nearly nine out of every 10 patients in whom TEER was used to repair their mitral valve,” said Makkar. “These strong safety and efficacy outcomes were validated, despite the advanced age and significant comorbidities of these patients.”
Using data from the Transcatheter Valve Therapy (TVT) registry—a jointly maintained database from the Society for Thoracic Surgery (STS) and the American College of Cardiology (ACC)—Makkar and his fellow investigators analysed 19,088 patients
who underwent TEER for moderate to severe isolated degenerative MR between January 2014 and June 2022.
The study’s primary endpoint was mitral regurgitation success, defined by investigators as moderate or better residual MR without narrowing of the mitral valve. Additional endpoints included death while hospitalised and within 30 days, and within one year of the procedure.
The study recorded that the patients’ average age was 82, and 49% were women. MR success was shown in 88.9% of patients, while at 30 days, the incidence of death was 2.7%, stroke was 1.2% and mitral valve reintervention was 0.97%. The lowest mortality rate was observed in patients who had both mild or less residual MR.
“For patients at elevated risk for surgery, TEER with the MitraClip device is a meaningful treatment option,” said Makkar. “The procedure is getting many patients back to a more
health and wellbeing,” said Lurz. “Building strong clinical evidence around the value of a procedure like tricuspid valve repair is critical, and the real-world outcomes of the bRIGHT study reinforce the safety and effectiveness of TriClip in reducing TR and improving quality of life.”
The study’s findings are limited by the fact that the study is a single-arm registry with no comparison to a conservative treatment group, the study team writes in JACC, as well as noting that they only report acute outcomes, and that longer-term outcomes are unknown.
The findings lead the study team to conclude that transcatheter tricuspid repair is “safe and efficient in treating significant TR in a diverse, real-world population”, and they note that the reduction in TR was associated with improvements in quality of life.
The real-world results from bRIGHT PAS add further data to the growing body of evidence for transcatheter tricuspid valve repair, following on from the results of the randomised TRILUMINATE pivotal trial, presented earlier in the year at the 2023 American College of Cardiology (ACC) annual scientific session (4–6 March, New Orleans, USA). The trial’s results showed that while the transcatheter system was effective in reducing TR and led to improvements in quality of life at one year, there were no significant differences in survival or heart failure hospitalisation between patients treated with the interventional approach or with medical therapy.
TriClip is approved for use in regions including Europe and Canada, but is an investigational device in the USA.
energetic life, and back to activities some have not been able to do for years.”
“Surgery is successful in nearly 100% of patients having degenerative mitral repair today in the USA, restoring normal life-expectancy in most patients,” said Joanna Chikwe (Cedars Sinai, Los Angeles, USA) a study author. “A heart team discussion is essential for patients deciding between surgery or interventional approaches, and we need randomised trials to inform these important decisions.”
Chikwe is principal investigator of the PRIMARY clinical trial—a multicentre, international trial— comparing the surgical approach for valve repair with the TEER procedure. The trial, funded by the National
Institutes of Health, is expected to complete enrolment in January 2026.

Treatment was
successful in nearly nine out of every 10 patients in whom transcatheter edge-to-edge repair was used to repair their mitral valve.”
RajMakkar
Structural Heart Interventions
Ten-year NOTION trial data provide important insights on long-term TAVI outcomes and valve durability
Ten-year data from the NOTION trial—the long-running clinical trial comparing outcomes among low-risk patients randomised to either transcatheter aortic valve implantation (TAVI) or surgical valve replacement (SAVR) for aortic stenosis—have shown that patients undergoing TAVI had a similar risk of all-cause mortality, stroke and myocardial infarction (MI) to those treated surgically.
THESE LONG-TERM DATA WERE reported by Troels Hojsgaard Jorgensen (Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark) at the European Society of Cardiology (ESC) congress (25–28 August, Amsterdam, The Netherlands), where he also told delegates that there was a higher risk of severe structural valve deterioration after SAVR, but a similar risk of bioprosthetic valve failure with the two approaches.

The performance of transcatheter technologies in the longer term is an important current area of focus as the procedure is increasingly moving toward a younger and lower risk patient population who are expected to live longer, and Jorgensen commented that there is still “much unknown” about the longterm clinical outcomes after TAVI.
“Over the past one and a half decades, numerous randomised clinical trials have been performed ensuring that TAVI is both safe and efficient across all the surgical risk strata,” Jorgensen said. “This means that TAVI has moved from being indicated initially in patients with prohibitive or high surgical risk to currently being indicated for patients above 75 years of age, or having a high surgical risk, and is considered equal to surgical aortic valve replacement in patients with an intermediate surgical risk.”
Initial trials looked at extreme- or highrisk patients, typically at an advanced age, meaning that there has been a lack of long-term follow-up data in this area. Trials in intermediate and low-risk groups offer the possibility to shed greater light on this issue, but to
date the best available data span eight years, with most current trials still far behind this point. NOTION is one of the frontrunners in addressing the question of long-term performance in a low-risk, all-comers population, and is the first to report at 10 years. Conducted at centres in Denmark and Sweden, the trial enrolled patients between 2009–2013 who were suitable for both TAVI and surgical valve replacement. Patients were required to be 70 years or older for inclusion in the trial. Those undergoing TAVI received the first-generation, self-expanding Corevalve (Medtronic) prosthesis, and patients undergoing SAVR received any surgical bioprosthesis at the discretion of the surgeon.
The study had a composite primary endpoint of all-cause mortality, stroke or MI after one year. In total 280 patients, with a mean age of 79 years, were randomised to receive TAVI or surgical intervention, with 139 ending up with a transcatheter valve, and 135 a surgical valve. Jorgensen commented that up to 50% of the patient population remained alive after
Outlining the results on clinical outcomes, Jorgensen reported that in terms of all-cause mortality there was no difference between the two groups, with a rate of 64% recorded in the SAVR arm at 10 years, compared to 62.7% in the TAVI group, while the composite rate of all-cause mortality, stroke and MI stood at 65.5% for both groups. Looking at complications individually,
Randomised trial “highly needed” to shed light on effectiveness of TAVI in bicuspid aortic stenosis patients
A DEDICATED, PROSPECTIVE randomised controlled trial (RCT) investigating the safety, efficacy and limitations of transcatheter aortic valve implantation (TAVI) versus surgical aortic valve replacement (SAVR) in different types of bicuspid aortic stenosis is “highly needed”, according to researchers writing in JACC:
Cardiovascular Interventions
The call comes in a viewpoint article, authored by Philippe Nuyens (Rigshospitalet, Copenhagen, Denmark) and colleagues, which aims to flag and address “important missing links in the current available evidence on TAVI in bicuspid aortic stenosis” as well as providing an initial impetus for the design of a trial comparing the two approaches.
Nuyens and colleagues write that the current available evidence on TAVI for the treatment of bicuspid aortic stenosis largely comes from observational or registry studies, which have mostly yielded reassuring results for TAVI— albeit based upon their inclusion of selected patients with favourable anatomy for transcatheter treatment.
“Another missing link,” they write, is that currently-available studies do not unravel which types or sizes of transcatheter valve are the better choices when treating specific bicuspid valves with TAVI. Additionally, they note that there is an absolute lack of long-term follow-up data as, among the currently available studies, there are nearly no reported outcomes beyond two years.
Despite this lack of data comparing
investigators saw comparable rates of cardiovascular death, stroke, transient ischaemic attack, and MI between the groups, though there were more patients in the surgical arm with new-onset atrial fibrillation (AF), and more patients in the TAVI arm needing a permanent pacemaker.
NOTION also sought to shed light on the question of valve durability, with investigators assessing this according to both bioprosthetic valve dysfunction, comprising structural valve deterioration, nonstructural valve deterioration, and bioprosthetic valve thrombosis or endocarditis, as well as bioprosthetic valve failure, which is made up of valve-related death, aortic valve reintervention, and severe haemodynamic structural valve dysfunction.
Structural valve deterioration was considered to be present if the patient had a transprosthetic mean gradient ≥20mmHg, an increase of 10mmHg from three months, or more than mild intraprosthetic aortic regurgitation. Jorgensen reported that the investigators found the risk of structural valve deterioration was increased in patients with a surgical bioprosthesis at 37.7%, compared to patients with a transcatheter heart valve, whose risk was 20.2%.
The investigators also assessed valve deterioration against a modified definition closer to that of the Valve Academic Research Consortium (VARC) 3 criteria, requiring both a high gradient alongside an increase of the gradient of at least 10mmHg or the development of more than mild intraprosthetic aortic regurgitation, which yielded structural valve deterioration rates of 21.6% for SAVR and 15.3% for TAVI respectively.
Detailing findings on the composite risk of bioprosthetic valve dysfunction, which Jorgensen described as “perhaps the most important risk factor if you look at it as a patient”, as it comprises the risk of valverelated death, the need for aortic valve reintervention, or the development of severe structural valve deterioration, there was a failure rate of 15.1% in the surgical arm, compared to 10.8% in the transcatheter arm, with no significant differences between the two groups overall, or for any of the individual endpoints apart from severe structural valve deterioration.
TAVI and SAVR in bicuspid aortic valve patients, TAVI for bicuspid aortic stenosis has received US Food and Drug Administration (FDA) approval.
“This approval may be somewhat premature and does not alter the fact that more prospective data are needed on the performance of TAVI versus SAVR in a non-selected, younger bicuspid aortic stenosis population,” Nuyens and colleagues write.
The paper goes on to present a study design proposal for a dedicated RCT comparing TAVI and SAVR in the bicuspid aortic stenosis patient population. “The goal of such a trial should be to provide insights on current aortic valve replacement practices in bicuspid aortic stenosis and to generate data and evidence that may influence guidelines and recommendations on the therapeutic choice for patients with bicuspid aortic stenosis and a longer life expectancy,” the paper’s authors state.
Long-term transcatheter valve durability will be a key question to
answer in any potential bicuspid aortic stenosis trial, Nuyens et al detail, adding that this will necessitate the enrolment of young and low-risk patients to guarantee for a large enough population of alive and “at risk” patients at five years and beyond. The study population should also represent an all-comers, young population with bicuspid aortic stenosis, similar to that seen in daily clinical practice.
A non-inferiority trial design is likely to be the most logical choice, the researchers state, as TAVI is not yet a fully established therapy to treat patients with bicuspid aortic stenosis with longer life expectancy.
The primary endpoint could be a composite of death, stroke and valverelated hospitalisation at five years, estimated to have event rates of 20% and 23% in the TAVI and SAVR treatment arms, respectively, the authors suggest, anticipating that a total sample size of 940 patients is likely to be required to produce an adequately powered trial.


DAVID HILDICK-SMITH
As one of the UK’s leaders in interventional cardiology, David Hildick-Smith (University Hospitals Sussex, Brighton, UK) has been a contributor to important research in areas including patent foramen ovale (PFO) closure, transcatheter aortic valve implantation (TAVI), left atrial appendage occlusion (LAAO) closure, and coronary angioplasty. Currently serving as the president British Cardiovascular Intervention Society (BCIS), Hildick-Smith tells Cardiovascular News about his life and career.
Why did you choose to become a doctor and why, in particular, did you choose to specialise in interventional cardiology?
I chose to be a doctor somewhat by default. I was good at science at school and I did not want to be a teacher or a proper scientist. I really wanted to be an astronomer and spend ages pondering the universe, but that was not very practical. I did medicine partly because all my sisters had also done that and partly as a way to get a degree of some sort, whether I went on to be a doctor or not.
Who were the biggest influences on your early career?
Two people really come to mind—Michael Petch and Len Shapiro, both at Papworth Hospital (Cambridge, UK). Michael Petch took a chance on appointing me to a good post when my CV looked rather lacklustre—I had been a journalist, an anaesthetist and a wanderer, so I was very lucky that he took a chance on me. I also really admired his clinical skills, and the way he could see about 50 patients in a clinic. Len Shapiro was just great at doing interventions. He did not always know quite how he had managed to make something work, but he usually managed it. He also had a lack of interest in petty bureaucracy or internal politics. He was interested in creativity and innovation and I aspired to have his interventional panache.
What has been the most important development in interventional cardiology during your career?
This one is easy—it has to be transcatheter aortic valve implantation (TAVI). It has been a massive transition for patients to be able now to have a valve replacement done through a 6mm blood vessel. Patients still find it amazing, and so do I if I sit down to think about it—the technological engineering is incredible. Patients often think we are the clever ones but it is the engineers who have done all the hard work. It has been amazing to be part of the TAVI experience right from the start in 2002. The excitement in 2007 when we did our first TAVI cases in Brighton was palpable.
How do you see TAVI evolving over the next 10 years?
TAVI will gradually become more routine, and lower risk, as valve profile, positioning and durability improve. However, rather paradoxically, the lower risk group of patients (aged 60 to 70) not uncommonly have the most problematic anatomy, and many of these would still be well advised to have surgery as a first option. It is hard to know how best
the increase in volume should be handled. TAVI requires a sizeable team to be able to deliver high volume and rapid turnover with good results. This means structural heart centres offering five days a week structural programmes, which is not yet common. There is also a discussion to be had about the number of patients with very limited life expectancy currently being referred for TAVI, and whether this is sensible.
What has been the biggest technological disappointment?
It would be a bit harsh to say it has been mitral intervention, but it has been mitral intervention! We did our first percutaneous mitral valve interventions nearly 10 years ago, but still the number overall are tiny, and no one has solved the big problems inherent in designing a valve that works. I remember in 2010 confidently predicting that mitral clip therapy only had five years left in it before being superseded by a full valve implant—how wrong I was! But, progress in the mitral valve area is definitely slow and there have been technologies that have come and gone. No one has yet fully solved the fact that the pesky anterior leaflet of the mitral valve has to get out of the way to let the blood out of the heart. Design by natural selection is still beating the best that human engineering can come up with—but it is also fantastic being part of the journey trying to solve these problems.
You have been instrumental in research in the field of PFO closure. In your view, should the procedure be restricted to patients under the age of 60?
It is of course, ludicrous to restrict the procedure on the basis of age alone. And in due course that will be challenged—maybe even in the courts! I understand why the age restriction is there, because that is the population studied, but the purpose of randomised trials is to try to answer a defined question in a rigorously clean set of circumstances, and then apply it to the wider population in an intelligent manner, case by case. There are very many people over the age of 60 who merit PFO closure and I have no doubt that as time progresses there will be more pressure from stroke physicians and neurologists to consider PFO closure in patients over the age of 60.
What important insights has the EBC MAIN trial (see page 19) added to our understanding of the treatment of left main bifurcation coronary artery disease?
I hope that EBC MAIN has been an important study for interventional cardiology. It is rare
FACT FILE
CURRENT APPOINTMENTS AND MEMBERSHIPS
Professor of Interventional Cardiology and Consultant Cardiologist, Sussex Cardiac Centre, Brighton, UK
President British Cardiovascular Intervention Society (BCIS), 2023/25
BCIS Structural Heart Disease Committee, Chair
European Association of Percutaneous Cardiovascular Interventions (EAPCI) Clinical Research Committee, Member National Institute for Health and Care Excellence (NICE), Interventional Cardiology Advisor CLINICAL TRIAL PARTICIPATION (SELECTED)
European Bifurcation Club Left Main Study (EBC MAIN)
SCOPE 2 randomised clinical trial
BHF PROTECT-TAVI randomised controlled trial
United Kingdom Transcatheter Aortic Valve Implantation (UK TAVI) Registry
that things are made better by being made complicated, and intervention of the left main bifurcation was in danger of being routinely overcomplicated. Cardiologists like to do challenging things but the overall patient outcomes are what matters most and there was a tendency to commit to a dual-stent strategy right from the beginning of a left main intervention, rather than take it step by step, stopping when you had a good result. It has been a problem for cardiology that the angiographically lovely result at the end of the procedure is not necessarily the one that serves the patient best in the long term. So EBC MAIN should make people pause before routinely considering a two-stent strategy, and that is a good thing.
What are your current research priorities?
I remain very interested in research around LAAO and PFO closure, both of which I think are going to expand greatly over the next ten years, particularly LAAO. In the coronary arena, I am struck by the continued price that is paid for stent implantation. It is not so uncommon to see patients coming back with a complication related to a stent that was implanted 10 years ago. The constant drip of inflammation related to the presence of a foreign body in the arteries is something which is going to get a great deal more attention in the next five years and I am currently looking to run a big European trial on drug-coated balloon technology for coronary bifurcations.
What has been the most important paper published in the past year?
Rather than pick out one I would simply say congratulations to anyone who has had an idea, written a protocol, sought funding, cajoled others into participating, collected the data and finally published their findings. It is a massive, sometimes thankless and solitary endeavour!
You are serving as president of BCIS— how important has membership of societies been to your career, and do you have any goals for your presidency?
I have always really enjoyed being part of BCIS in order to try to have a say in influencing development of services. I have also contributed to many other societies to try to bring about change. For BCIS, the biggest challenge professionally for cardiologists is the primary angioplasty service. It has been extremely beneficial for patients, but providing the service is bringing many cardiologists and allied health professionals to their knees. We need to ensure that services
are sustainable and are staffed by enough people to make rotas survivable for a career. Regional network planning of rotas should address minimum numbers for sustainability. Given the known harm of night-time call, days off after being on call should be written into job plans in accordance with BCIS guidance.
There is an unfortunate exodus of talented and experienced older cardiologists. Much of this of course relates to the arduous nature of being on call. In order to continue to attract the best people into cardiology and the cath lab environment, we need to make sure that they can see something other than an entire career of night-time primary angioplasty ahead of them. We need to discuss rota management such that colleagues can, for example, share an on-call slot aged 55 and, for example, come off night time on call aged 60 (and perhaps do weekend days instead). Otherwise we will continue to lose older cardiologists prematurely, while failing to attract some of the brightest young trainees into the specialty.

As regards career development, every
cardiologist needs to be able to see opportunities ahead. Over the last decade, most interventional opportunities have been focussed in regional hospitals. In district hospitals, opportunities have been fewer. There are opportunities, though, to introduce innovative treatments to a district hospital setting. Stroke intervention, pulmonary embolectomy, renal denervation, peripheral angioplasty or coronary sinus technologies, for example, should all be available as options that need to be spread through many hospitals in the country, just as some other treatments need to be available in all surgical centres.
What can interventional cardiology do to attract young physicians to the field?
See answer above! Of course the daytime work is extremely rewarding and we are very lucky in the work that we do. We still have the respect of the patients and most of them are extremely grateful for our efforts. The nighttime work is a problem. It definitely puts off some excellent trainees, and I am not surprised. I do think there are options for limiting the
amount of work that consultants do at night. Being regularly woken from sleep to go in to the hospital is a peculiar form of torture, and it is definitely bad for your health. As a trainee you want to see some “open road” in front of you, when things will be easier, and worth working for. At the moment we do not have that balance right.
Outside of medicine, what are your hobbies and interests?
I like music, I play the guitar and piano a fair bit and have a Spotify channel. I also like to play poker, though I am pretty bad at it as I do not have a poker face. I play cricket about once a year though I like to think it’s more often. I garden and like to pretend that I live in a Mediterranean climate by trying to grow peaches and lemons (and failing) and I have inherited a small pond which takes my time as I do battle with the algae and try to get the tiny solar pump to work. I like to walk, spend time with my family, and try to spend time not pondering the world too much but applying the power of now (and usually failing too!).
"It has been amazing to be part of the TAVI experience right from the start in 2002. The excitement in 2007 when we did our first TAVI cases in Brighton was palpable.”
Nitrate reduces contrastinduced necropathy during coronary angiography
Inorganic nitrate reduces contrast-induced nephropathy (CIN), improves renal outcomes and reduces cardiac events compared to placebo in patients at risk of renal injury undergoing coronary angiography for acute coronary syndrome.
This was the conclusion presented by Dan Jones (Queen Mary University of London, London, UK), who reported the findings of NITRATE-CIN, a trial examining the efficacy of administering inorganic nitrate for the prevention of CIN in patients with nonST elevation acute coronary syndrome (NSTE-ACS) referred for invasive coronary angiography and at risk of CIN, at the European Society of Cardiology (ESC) congress (25–28 August, Amsterdam, The Netherlands).
CIN refers to a deterioration in renal function after contrast exposure. Older age, heart failure, chronic kidney disease (CKD) and diabetes are independent predictors of CIN following coronary angiography for acute coronary syndromes. The complication is associated with longer hospital stays, an increased risk of myocardial infarction (MI) and higher mortality.
ESC guidelines highlight that patients with CKD have been excluded from randomised trials on myocardial revascularisation and that additional randomised evidence is needed on optimal strategies for CIN prevention. Research has suggested that nitric oxide is deficient in CIN; therefore, strategies to replace nitric oxide might be of benefit. Inorganic nitrate is metabolised in the body to deliver nitric oxide to areas of the body and has shown renoprotective effects in preclinical studies.
NITRATE-CIN was a double-blind, randomised, placebo-controlled trial conducted at Queen Mary University of London and St Bartholomew's Hospital in London, UK. Patients were randomised in a 1:1 ratio to once daily potassium nitrate (12mmol) or placebo
(potassium chloride) capsules for five days
In the trial, Jones reported at ESC 2023, risk of CIN was defined as an estimated glomerular filtration rate (eGFR) <60 ml/min or two of the following: diabetes, liver failure (cirrhosis), age >70 years, exposure to contrast in the seven days prior to angiography, heart failure (or left ventricular ejection fraction <40%), and concomitant renally active drugs.

The primary endpoint was the incidence of CIN (³0.3mg/dl or ³26.5mmol/L increase in creatinine within 48 hours or ³1.5 times within one week), as defined by the Kidney Disease Improving Global Outcomes (KDIGO) criteria for acute kidney injury. Secondary outcomes included renal function (eGFR) at three months, rates of procedural MI and major adverse cardiac events (MACE, a composite of death, non-fatal MI and unscheduled revascularisation) at 12 months.
ECLS not shown to improve survival in patients with acute MI complicated by cardiogenic shock
Early extracorporeal life support (ECLS) does not improve survival in patients with acute myocardial infarction (MI) complicated by cardiogenic shock who are scheduled for early revascularisation.
THIS WAS THE HEADLINE finding from the ECLS-SHOCK trial, presented during a Hot Line session at the European Society of Cardiology (ESC) congress (25–28 August, Amsterdam, The Netherlands).

According to the trial’s principal investigator, Holger Thiele (Leipzig Heart Centre, Leipzig, Germany), the findings may lead to the discontinuation of the routine use of ECLS therapy, in this setting.
Standard care for CIN prevention matched ESC recommendations (hydration before and after coronary angiography, use of low-osmolar contrast media).
Over a three-year period, a total of 640 patients were randomised: 319 received inorganic nitrate and 321 received placebo. The mean age of trial participants was 71 years, 73.3% were men, 75.2% were Caucasian, while 45.9% had diabetes, 56% had chronic kidney disease (eGFR <60 ml/min) and the mean Mehran score was 10. The median follow up was one year.
Jones reported that inorganic nitrate treatment significantly reduced CIN rates (9.1%) versus placebo (30.5%; p<0·001). The difference persisted after adjustment for baseline creatinine concentration and diabetes status (odds ratio [OR] 0.21; 95% confidence interval [CI] 0.13 to 0.34). Secondary outcomes were improved with inorganic nitrate, with lower rates of procedural myocardial infarction (2.7% vs. 12.5%; p=0.003), improved three-month renal function (between group change in eGFR 5.17 [interquartile range 3.94-7.39]) and reduced one-year MACE (9.1% vs. 18.1%; p=0.001) versus placebo.
“In patients at risk of renal injury undergoing coronary angiography for acute coronary syndrome, dietary inorganic nitrate reduced CIN compared to placebo,” said Jones. “This corresponded to improved renal outcomes at three months and MACE at 12 months. These findings could have important implications for health systems by reducing the burden of CIN and the associated prolonged admissions, dialysis, and significant costs. Further studies powered for adverse cardiac events are needed to confirm these findings.”
ECLS-SHOCK was the first randomised trial to investigate the effect of ECLS on mortality in acute MI complicated by cardiogenic shock.
A total of 420 patients with acute MI and cardiogenic shock scheduled for early revascularisation were enrolled from 44 centres in Germany and Slovenia. The median age of participants was 63 years and 19% were women. Patients were randomly assigned to early ECLS plus usual medical treatment (ECLS group) or usual medical treatment alone (control group).
The trial’s primary endpoint was all-cause death at 30 days, whilst secondary endpoints included length of mechanical ventilation, time to haemodynamic stabilisation and need for renal replacement therapy. Safety endpoints included moderate or severe bleeding and peripheral vascular complications requiring intervention
A total of 417 patients were included in the final analyses, in which it was reported that the primary endpoint of all-cause death at 30 days occurred in 100 of 209 patients (47.8%) in the
ECLS group and in 102 of 208 patients (49%) in the control group.
The median duration of mechanical ventilation was longer in the ECLS group: seven days in the ECLS group versus five days in the control group. The time to haemodynamic stabilisation and rates of renal replacement therapy were similar between treatment groups.
Regarding safety endpoints, Thiele reported that moderate or severe bleeding occurred more frequently in the ECLS group: 23.4% of patients in the ECLS group versus 9.6% of patients in the control group, and peripheral vascular complications requiring intervention occurred also more often in the ECLS group: 11% of patients in the ECLS group versus 3.8% of patients in the control group.
“The results of ECLSSHOCK demonstrated no reduction in 30-day mortality with early ECLS therapy and an increase in complications,” Thiele said. “The findings may lead to the discontinued routine use of these devices in clinical practice.”
These findings could have important implications for health systems by reducing the burden of CIN.”
Biolimus-coated balloon fails to match paclitaxel for in-stent restenosis
Results of the first study of a first-generation drug-coated balloon (DCB) with a biolimus coating for the treatment of in-stent restonsis—REFORM—have shown that the device failed to meet non-inferiority compared to a paclitaxel-coated device.
Robert Byrne (RSCI University of Health Sciences, Dublin, Ireland) reported the findings of the study at EuroPCR 2023 (16–19 May, Paris, France). DCBs are seen as an appealing treatment option for in-stent restenosis as once the drug has been delivered using the device, no permanent metallic structure is left behind in the vessel, unlike with a stent.
“Patients who have undergone PCI [percutaneous coronary intervention] with a drug-eluting stent still fall foul to in-stent restenosis,” Byrne commented in his presentation, noting that this outcome occurs in roughly 3–5% of patients. “Guideline recommended treatments include repeat stenting with drug-eluting stents and angioplasty with a drug-coated balloon.”
To date, paclitaxel has been the coating of choice for the majority of DCBs due to its lipophilic properties that assist its transfer and retention at
the vessel wall. Limus-based drugs such as sirolimus have supplanted paclitaxel in drug-eluting stents, where they have shown superior safety and efficacy, as well as having a wider therapeutic range than paclitaxel, but, a crucial difference in DCB-usage has been that the drug is absorbed more slowly into the vessel wall.
The prospective, randomised, noninferiority trial pitted the Biolimus A9coated balloon (Biosensors) against the SeQuent Please (B Braun) paclitaxel DCB, over a primary endpoint of percentage in-segment diameter stenosis at six months.
The REFORM study screened a total of 351 patients for inclusion, Byrne reported at EuroPCR, with 135 randomised to receive the Biolimus-coated device, and 66 randomised to receive the SeQuent Please, paclitaxel
-coated device.
Detailing the results of the study, Byrne reported that percentage insegment diameter stenosis at six months was 41.8% with the Biolimus-coated balloon versus 31.2% for the paclitaxelcoated balloon, meaning that the investigational device did not meet its criteria for non-inferiority.
Commenting on further endpoints of note, Byrne stated that late lumen loss recorded with the investigational device averaged 0.5mm, compared to 0.2mm with the comparator—the lowest of any prior study in in-stent restenosis using the SeQuent Please device—while binary restenosis stood at 32.2% and 11.3% in each cohort, respectively.
Offering up some possible explanations for the findings, which he claimed were “not in line with expectations”, Byrne said: “One might be a difference in the rate of negative late lumen loss or positive vessel remodelling which was observed more frequently with the paclitaxel-coated device, as compared with the Biolimus-coated device.
“In addition, one might comment that the performance of the control device, the Sequent Please was excellent in this study and out-performed that in other trials with a late lumen loss of 0.2.”
Furthermore,
Three-year EBC MAIN results demonstrate need for pragmatism in left main bifurcation stent strategy
Operators performing percutaneous coronary intervention (PCI) in bifurcation left main stem stenosis should not decide on the number of stents they intend to use prior to the procedure, delegates at EuroPCR 2023 (16–19 May, Paris, France) were told.
THIS WAS THE MESSAGE OF DAVID Hildick-Smith (University Hospitals Sussex, Brighton, UK), who delivered three-year findings of the EBC MAIN study during a late-breaking trial session on day one of the EuroPCR meeting. EBC MAIN is a randomised comparison of a provisional versus a systematic dual stent strategy for true bifurcation left main coronary artery disease, involving 467 patients at over 30 centres across Europe. Patients were randomised either to receive the stepwise provisional or dual-stent PCI strategy. The study was initiated by the European Bifurcation Club and supported by Medtronic, whose Resolute Onyx drug-eluting stent platform was used throughout the study.

One-year results of the trial, reported in 2020, indicated that there was no difference in terms of the clinical outcomes of death, myocardial infarction (MI) or target lesion revascularisation (TLR) between the planned single stenting and the upfront use of twostent techniques. The study also showed that 22% of patients randomised to a planned single-stent strategy were ultimately treated with two stents, a fact that was highlighted by Hildick-Smith in his presentation. Outlining the trial’s three-year results, HildickSmith reported that there was no difference in the primary endpoint of death, MI and TLR between the two groups (23% in the provisional cohort vs. 29% in

the dual-stent cohort, p=0.02) at 36 months, though he did note that there was almost twice as much TLR in the systematic dual stent group (8% vs. 14%).
“It is not necessary to decide before you do the case how many stents you are going to put in. If I was allowed only one message from this trial, that would be it,” said Hildick-Smith of the results, with reference to the significance of the 22% of patients in the provisional group requiring a second stent. The results, he said, reaffirm the provisional approach as the “technique of choice” for bifurcation left main stem stenosis.
Byrne noted that in the first-generation Biolimus-coated balloon, the crystalline coating had a crystal size of around 10 microns, whereas a second-generation iteration has been developed with a smaller crystal size which he said can result in higher tissue levels in the same preclinical models and has received clinical trial approval in China.
“Initial data have become available from the BIO-RISE trial in patients with small vessels showing that it outperforms balloon angioplasty, and a trial in in-stent restenosis is ongoing and is expected to report this year,”
Byrne commented.
In-segment diameter stenosis at six months
“The decision to put two stents in is very commonly taken at a very early stage,” Hildick-Smith commented to Cardiovascular News, in relation to his headline message that operators should not approach left main bifurcation cases with a predetermined single- or double-stent strategy in mind.
“You will see at live case meetings and other discussions, people will show an angiogram and IVUS [intravascular ultrasound] images and the comment will be made, ‘I am going to do a twostent strategy right from the start’, and it is slightly frustrating to hear that because, actually, the evidence that we have shown is that you do not need to make that judgement up front.”
Hildick-Smith commented that, in EBC Main, among patients who were randomised to the provisional strategy, only one out of five of them required that second stent. “If you take it logically step by step, and ask at each step, ‘have I done enough [or] do I need to do some more?’ then actually you can stop sooner than you realise at the outset, so it is absolutely not necessary to decide before you do the procedure how many stents you are going to put in.”
It is not necessary to decide before you do the case how many stents you are going to put in. If I only was allowed one message from this trial, that would be it.”Robert Byrne Biolimuscoated balloon 41.8% Paclitaxelcoated balloon 31.2% David Hildick-Smith


No increased risk of hand dysfunction from distal compared to proximal radial artery access for cardiac procedures
A randomised controlled trial comparing the safety of distal versus proximal radial artery access for cardiac catheterisation has concluded that there is no increased risk of hand dysfunction or radial artery occlusion at one year.
This is among the headline findings from the DIPRA—Distal versus proximal radial artery access for cardiac catheterization and intervention—study, presented as latebreaking clinical research at the Society for Cardiovascular Angiography & Interventions (SCAI) 2023 scientific sessions (18–20 May, Phoenix, USA).
The single-centre, randomised trial evaluated outcomes of hand function and effectiveness of conventional proximal radial artery access compared to distal radial artery access for cardiac
catheterisation.
Current guidelines for patients undergoing percutaneous intervention recommend proximal artery access, one complication of which is radial artery occlusion, which can compromise the access of the artery for future coronary bypass surgery, dialysis or other cardiovascular procedures.
The DIPRA study randomised 300 patients 1:1 to cardiac catheterisation
Imaging technology reduces contrast use during PCI
The use of an image-guidance system that overlays angiographic images on live fluoroscopic images has been shown to reduce the amount of contrast used during percutaneous coronary intervention (PCI), trial data shared at EuroPCR 2023 (16–19 May, Paris, France) indicate.
DYNAMIC CORONARY ROADMAP (DCR) is an imaging technology developed by Philips and is designed to reduce total iodinated contrast media volumes administered during PCI procedures. The software is intended to provide a real-time and dynamic angiographic roadmap of coronary arteries, which is automatically generated from previously acquired coronary angiograms during the same procedure.
Javier Escaned (Hospital Clinico San Carlos, Madrid, Spain) delivered results of the Dynamic Coronary Roadmap for Contrast Reduction—DCR4Contrast— trial during a late-breaking trial session at EuroPCR, where he revealed that the use of the system resulted in a reduced use of contrast media on average by 28.8% compared to PCI using angiography alone (95% Confidence Interval [CI]: 18.9%, 38.2%).

Additionally, the trial found that the use of DCR was able to reduce the number of angiograms per PCI procedure on average by three runs based on a PCI procedure with an average of 11 runs, or a 26.3% reduction (95% CI: 16.8%, 35.1%).
Running in centres across the USA, Europe and Israel, DCR4Contrast randomised a total of 371 patients who were undergoing both ad hoc and planned PCI procedures. Patients were stratified according to the number of vessels that needed to be treated.
through either distal or proximal radial artery access. The primary endpoint was change in hand function from baseline to one year. Hand function was a composite of the QuickDASH questionnaire, hand grip test, and thumb-forefinger pinch test. Secondary endpoints included access feasibility, radial artery patency, and complications.
Of 216 patients who completed oneyear follow-up, 112 were randomised to distal radial artery access and 104 to proximal radial artery access. Both groups had similar access site bleeding rates (distal 0% vs. proximal 1.4%; p=0.25).
Six distal radial artery patients failed access compared to two proximal radial artery patients. Radial artery occlusion occurred in one proximal patient versus two in distal access. At one year, there was no significant difference in the change of hand function, in hand grip
(distal 0.7 [-3, 4.5] vs. proximal 1.3 [-2, 4.3] kg; p=0.57), pinch grip (distal -0.1 [-1.1, 1] vs. proximal -0.3 [-1, 0.7] kg; p=0.66), and QuickDASH (distal 0 [-6.6, 2.3] vs. proximal 0 [-4.6, 2.9] points, p=0.58). The composite of hand function was comparable between
Patients within the DCR group underwent PCI procedures where DCR was used to guide the advancement of coronary wires, balloons, stents and other PCI diagnostic or therapeutic devices. Patients assigned to the control group underwent PCI without DCR support following standard care.
Speaking to Cardiovascular News, Escaned said that the DCR technology potentially has a particular benefit among patients undergoing complex PCI, as well as those with renal impairment.

“There is a great interest nowadays in so-called ultra-low contrast PCI, which is basically the skillset needed to reduce dependence of contrast administration particularly in complex patients during PCI,” said Escaned, highlighting that the issue features in a chapter in this year’s PCR-EAPCI textbook, published to coincide with EuroPCR.
“This feels like a very promising tool for this purpose, because it reduces radiation, the amount of contrast, and also it helps in transitioning from a culture of requiring the use of repeated vessel pacification with contrast, to something that relies more on a different technology.”
Escaned said that the system is relatively simple to use, given its use of existing techniques that most interventional cardiologists will be familiar with.
Speaking during a press conference at the EuroPCR meeting, William Wijns (Lambe Institute for Translational Medicine, Galway, Ireland) described the findings of the study as “really important” due to the potentially life-threatening impact of contrast-induced necropathy and other side effects related to contrast usage.

proximal and distal access at one year.
“We know that radial artery occlusion is a potential complication of repeated heart catheterisations through the wrist. We also know that distal radial artery access in the base of the thumb carries a lower risk for this complication,” said Karim Al-Azizi (Baylor Scott & White Health, Plano, USA), lead author of the study. “The one-year safety results presented at SCAI are reassuring and offer physicians an alternative approach for patients who need radial access, such as patients with chronic kidney disease for dialysis access or coronary artery disease patients who need bypass grafting.”
Immediate PCI of
lesions
IMMEDIATE MULTIVESSEL
percutaneous coronary intervention (PCI) is noninferior to staged multivessel PCI for reducing death and ischaemic events in patients with ST-segment elevation myocardial infarction (STEMI) and multivessel coronary artery disease.
This was the headline finding of the MULTISTARS AMI trial, presented during a Hot Line trial session at the European Society of Cardiology (ESC) congress (25–28 August, Amsterdam, The Netherlands).
Barbara Stähli (University Hospital Zurich, Zurich, Switzerland) reported that at one-year, the trial’s primary endpoint, a composite of all-cause death, non-fatal myocardial infarction (MI), stroke, unplanned ischaemia-driven revascularisation, or hospitalisation for heart failure, occurred in 8.5% of patients randomised to immediate PCI, compared to 16.3% in the staged group.
The trial enrolled a total of 840 patients, who were randomised 1:1 to either the staged or the imediate approach.
“MULTISTARS AMI addresses the clinically important question of the optimal timing for a complete revascularisation of patients with STEMI and multivessel coronary artery disease,” said Stähli. “The trial has implications for clinical practice, as it demonstrated that immediate PCI of nonculprit lesions is as effective and safe as a staged procedure. Results were generally consistent across prespecified key subgroups, particularly among women and men, young and older patients, and patients with or without diabetes.”
We know that radial artery occlusion is a potential complication of repeated heart catheterisations through the wrist.”
non-culprit
“as effective as staged procedure”
Gut feeling: Studies find link between diet, gut health and atherosclerosis
Two recent studies have shed new light on the connection between diet, gut health and atherosclerosis, potentially paving the way, according to researchers, for targeted interventions among individuals who may be at risk for developing heart disease.
“The gut is the dietary window to the body,” said Srinivasa Reddy (University of California Los Angeles [UCLA], Los Angeles, USA), corresponding author on a study published in the Journal of Lipid Research, in which it was determined that derivatives of natural emulsifiers such as phospholipids found in high-fat, high-cholesterol diets can promote atherosclerosis via gut bacteria interactions with the immune system.
To study the connection between diet and atherosclerosis, the researchers used a mouse model that recapitulates the levels of low-density lipoprotein, or “bad cholesterol,” seen in atherosclerosis patients and that lack the specific enzyme involved in the generation of pro-inflammatory derivatives of natural emulsifiers in the intestinal lining cells. Using this model, the researchers found that on a high-fat, highcholesterol diet, the cells that line the small intestine produce phospholipids that make the intestinal lining more susceptible to invasion by gut bacteria.
“The normal defences for intestinal lining cells to keep bacteria in the lumen of the intestine are reduced when they take up large amounts of cholesterol and fat,” Alan Fogelman, a professor of medicine at UCLA and project supervisor, said. “This also results in bacteria being able to come in direct contact with the cells lining your intestines called enterocytes. Without those defences, this results in more bacterial products, like bacterial cell membranes that contain a toxin called endotoxin, getting into the bloodstream to cause inflammation.”

Release of bacterial products from the gut into the blood stream sounds an alarm in the immune system, which deploys immune cells into the blood to eliminate the potential threat.
“People who are obese and people eating high-fat, high-cholesterol diets have higher levels of endotoxin in their blood,” Fogelman continued. “It is not at the level of causing sepsis, but it causes a low level of inflammation. When the cholesterol and fat come into the mix, the endotoxin kind of turns up the thermostat on inflammation and that accelerates atherosclerosis
Multiple unhealthy traits linked to earlier incidence MI or stroke
MIDDLE-AGED ADULTS WITH three or more unhealthy traits including slightly high waist circumference, blood pressure, cholesterol and glucose have myocardial infarction (MI) and stroke two years earlier than their peers, according to research presented at the European Society of Cardiology (ESC) congress (25–28 August, Amsterdam, The Netherlands).
The results highlight the need for screening for individuals who may be at risk for metabolic syndrome, an
and leads to increased heart attacks and strokes.”
The team is looking for ways to reduce the phospholipid derivatives that cause endotoxin to enter the blood stream. One method they have explored previously is using a mimetic of high-density lipoprotein, sometimes called good cholesterol.
“We created transgenic tomatoes in our lab that mimic the good cholesterol, high-density lipoprotein,” Arnab Chattopadhyay (UCLA, Los Angeles, USA) project scientist and lead author of the study, said. “These tomatoes, when added to a highfat, high-cholesterol diet, help lower cholesterol and triglycerides and also lower the inflammatory derivatives of the phospholipids.”
The team said that this method of lowering cholesterol levels and triglycerides could be beneficial to obese individuals at risk for inflammatory diseases such as atherosclerosis, arthritis, lupus, multiple sclerosis and more.
Further, unconnected, research from Sweden has also shown how gut health can impact the development of atherosclerosis, with researchers from Uppsala and Lund universities in Sweden reporting in Circulation a link between certain levels of gut bacteria and coronary plaques.
The study was based on analyses of gut bacteria and cardiac imaging among 8,973 participants aged 50‒65 from Uppsala and Malmö without previously known heart disease. They were all participants in the Swedish CArdioPulmonary bioImage Study (SCAPIS).
“We found that oral bacteria, especially species from the Streptococcus genus, are associated with increased occurrence of atherosclerotic plaques in the small arteries of the heart when present in the gut flora.
investigator in the study has stated.
Lena Lönnberg (Västmanland County Hospital, Västerås, Sweden) presented the findings of the population-based study, which investigated the link between asymptomatic metabolic syndrome in midlife and cardiovascular disease and death.
Researchers enrolled 34,269 adults in their 40s and 50s who attended a cardiovascular screening programme from 1990 to 1999 in the Swedish county of Västmanland, who were assessed at a primary healthcare centre for measurements including their height, weight, blood pressure, total cholesterol, blood glucose, and waist and hip circumference. They also completed a questionnaire about lifestyle habits, previous history of cardiovascular disease and diabetes, and socioeconomic factors such as education.
Species from the Streptococcus genus are common causes of pneumonia and infections of the throat, skin and heart valves. We now need to understand whether these bacteria are contributing to atherosclerosis development,” stated Tove Fall (Uppsala University, Uppsala, Sweden) who coordinated the study together with researchers from Lund University.
Advancements in technology have enabled largescale deep characterisation of bacterial communities in biological samples by sequencing the DNA content and comparing it to known bacteria sequences.
Additionally, improvements in imaging techniques have enabled the detection and measurement of early changes in the small vessels of the heart. The SCAPIS study represents one of the largest collections in the world of both these kinds of data. In this study, scientists investigated the links between the gut microbiota and the build-up of fatty deposits in the arteries of the heart.
“The large number of samples with high-quality data from cardiac imaging and gut flora allowed us to identify novel associations. Among our most significant findings, Streptococcus anginosus and S. oralis subsp. oralis were the two strongest ones,” said Sergi Sayols-Baixeras, lead author from Uppsala University.
The research team also found that some of the species linked to the buildup of fatty deposits in heart arteries were linked to the levels of the same species in the mouth. This was measured using faecal and saliva samples collected from the Malmö Offspring Study and Malmö Offspring Dental Study. Furthermore, these bacteria were associated with inflammation markers in the blood, even after accounting for differences in diet and medication between the participants who carried the bacteria and
“We have just started to understand how the human host and the bacterial community in the different compartments of the body affect each other. Our study shows worse cardiovascular health in carriers of streptococci in their gut. We now need to investigate if these bacteria are important players in atherosclerosis development,” noted Marju Orho-Melander (Lund University, Lund, Sweden), one of the senior authors of the study.
Individuals were classified as having metabolic syndrome if they had three or more of the following: waist circumference of 102cm or above for men and 88cm or above for women; total cholesterol 6.1mmol/l or above; 130mmHg or higher systolic blood pressure and/or 85mmHg or higher diastolic blood pressure; fasting plasma glucose 5.6mmol/l or higher.
Those with metabolic syndrome were matched for age, sex and date of health examination to two individuals without metabolic syndrome who served as controls. Data on cardiovascular events (MI and stroke) and death were collected from national and local registers. Researchers analysed the associations between midlife metabolic syndrome and non-fatal cardiovascular events and all-cause mortality.
A total of 5,084 individuals (15%) met
the criteria for metabolic syndrome and a control group of 10,168 individuals without metabolic syndrome was identified. Some 47% of participants were women. During a median followup of 27 years, 1,317 (26%) participants with metabolic syndrome died compared with 1,904 (19%) controls—meaning that those with metabolic syndrome were 30% more likely to die.
Lönnberg said: “The results underline the importance of early detection of risk factors through health screening programmes so that preventive actions can be taken to prevent heart attack, stroke and premature death. As a general rule of thumb, even if you feel well, check your blood pressure every year, avoid smoking, keep an eye on your waist circumference and last, but definitely not least, be physically active every day.”
“The gut is the dietary window to the body”
STEMI patients “lost years of life” due to care delays during COVID-19 lockdowns
Patients with ST-elevation myocardial infarction (STEMI) during the first COVID-19 lockdown in the UK and Spain are predicted to live 1.5 and two years less, respectively, than their preCOVID-19 counterparts.
THAT IS ACCORDING TO THE findings of a study published in European Heart Journal–Quality of Care and Clinical Outcomes, authored by William Wijns (Lambe Institute for Translational Medicine, University of Galway, Galway, Ireland). The additional costs to the UK and Spanish economies are estimated at £36.6 million (€41.3 million) and €88.6 million, respectively, largely due to absence from work.

“Restrictions to treatment of lifethreatening conditions have immediate and long-term negative consequences for individuals and society as a whole,” said Wijns. “Back-up plans must be in place so that emergency services can be retained even during natural or health catastrophes.”
Delays to percutaneous coronary intervention (PCI) and the resulting lack of oxygen, lead to irreversible damage to the heart. During the first wave of the pandemic, about 40% fewer heart attack patients
went to hospital as governments told people to stay at home, people were afraid of catching the virus, and some routine emergency care was stopped. Compared to receiving timely treatment, those who stayed at home were more than twice as likely to die, while those who delayed going to the hospital were nearly twice as likely to have serious complications that could have been avoided.
The study estimated the long-term clinical and economic implications of reduced treatment during the pandemic in the UK and Spain. The researchers compared the predicted life expectancy of STEMI patients during the first lockdown with those who had a heart attack at the same time in the previous year. Researchers also compared the cost of STEMIs during lockdown with the year before.
A model was developed to estimate long-term survival, quality of life and costs related to STEMI. The UK analysis compared the period 23 March (when lockdown began) to 22
April 2020 with the equivalent time in 2019. The Spanish analysis compared March 2019 with March 2020 (lockdown began on 14 March 2020).
Survival projections considered age, hospitalisation status and time to treatment using published data for each country. For example, using published data, it was estimated that 77% of STEMI patients in the UK were hospitalised prior to the pandemic compared with 44% during lockdown. The equivalent rates for Spain were 74% and 57%. The researchers also compared how many years in health were lost for STEMI patients before and during the pandemic.
The cost analysis focused on initial hospitalisation and treatment, follow-up treatment, management of heart failure and productivity loss in patients unable to return to work. For example, the cost applied to a STEMI admission with PCI was £2,837 in the UK and €8,780 in Spain. Heart failure costs were estimated at £6,086 in year one and £3,882 in all subsequent years for the UK. The equivalent figures for Spain were €3,815 (year one) and €2,930 (each subsequent year).
The analysis predicted that patients who had a STEMI during the first UK lockdown would lose an average of 1.55 years of life compared to patients presenting with a STEMI before the pandemic. In addition, while alive, those with a STEMI during lockdown were predicted to lose approximately one year and two months of life in perfect health. The equivalent figures for Spain were 2.03 years of life lost
NACMI registry finds increased incidence of clotting among STEMI patients with COVID-19

Analysis from The North American COVID-19 STEMI (NACMI) registry, presented as late-breaking clinical research at the Society for Cardiovascular Angiography & Interventions (SCAI) 2023 scientific sessions (18–20 May, Phoenix, USA), has shown that patients with an ST-elevated myocardial infarction (STEMI) and COVID-19 had a significant amount of clotting in their arteries both before and after intervention.
CLOTS WERE SEEN IN MULTIPLE ARTERIES in close to 30% of patients, a phenomenon observed in less than 5% of patients with heart attacks who do not have COVID-19, the researchers have reported.
In the USA, someone experiences a heart attack every 40 seconds. Of these patients, more than 25% will experience STEMI caused by the sudden, total blockage of a coronary artery. Pre-COVID-19 mortality in STEMI patients was below 5%, and previous NACMI research has shown that mortality jumps to between 20% to 25% in patients who present with COVID-19 and a heart attack.
In this blinded angiographic analysis, sites sent angiograms to the Cardiovascular Imaging Research Core Lab (Vancouver, Canada), where they were assessed for quantitative coronary angiography percent diameter stenosis, thrombolysis in myocardial infarction (TIMI) flow, myocardial blush grade (MBG) and thrombus grade burden (TGB).
Percutaneous coronary intervention (PCI) was
classified as unsuccessful if there was residual diameter stenosis >50% and/ or <TIMI 2 flow and/or untreated complication. Multivessel thrombotic disease and stenotic disease was defined as thrombus grade burden >0 and diameter stenosis > 50% in > 2 arteries, respectively.
Angiograms of 234 patients from 17 sites (12 in USA, and five in Canada)
and around one year and seven months of life in perfect health lost.
In the UK, the extra cost of one STEMI during the pandemic, compared to before, was £8,897 which included £214 for the National Health Service and £8,684 in work absenteeism. Based on an incidence of 49,332 STEMIs per year, reduced access to PCI during the first month of lockdown was projected to cost an extra £36.6 million (€41.3 million) over the lifetime of these patients.
For Spain, the extra cost per STEMI during lockdown was estimated at €20,069. Based on an annual STEMI incidence of 52,954 STEMIs, reduced access to PCI during March 2020 was projected to cost an additional €88.6 million over these patients’ lifetimes. Work absenteeism was the main contributor, costing an extra €23,224 per patient (€81,062 prevs. €104,286 post-pandemic). However, this was partially offset by lower costs of heart failure hospitalisations since more STEMI patients died during lockdown.
Wijns said: “The findings illustrate the repercussions of delayed or missed care. Patients and societies will pay the price of reduced heart attack treatment during just one month of lockdown for years to come. Health services need a list of lifesaving therapies that should always be delivered, and resilient healthcare systems must be established that can switch to emergency plans without delay. Public awareness campaigns should emphasise the benefits of timely care, even during a pandemic or other crisis.”
were analysed. High thrombus grade burden was observed in 74% of all patients pre-intervention and 29% of patients post intervention. A high proportion of patients (19%) did not have culprit lesions (locations inside the arteries readily identified by treating physicians) suggesting other mechanisms for heart attack may be at play in this patient population.
Core lab identified stent thrombosis (clotting of previously placed stents) in 12% of the entire cohort —a frequency that has never been observed in other STEMI cohorts. Of the 49 patients, Core lab identified PCI failure rates were 34% which a high complication rate of 23%, mostly related to thrombus.
“COVID-19 is a pro-inflammatory, clot forming disease and we now see its effect in the coronary arteries,” said Payam Dehghani (University of Saskatchewan, Saskatoon, Canada). “These new insights point to the need for clinicians to be meticulous with blood thinning strategies, early interventions and patient follow-up.”
The NACMI registry is a collaboration between SCAI, the American College of Cardiology (ACC) and the Canadian Association of Interventional Cardiology (CAIC). The registry was established in 2020 with the aim to define baseline characteristics and management strategies and outcome data for COVID-19 patients presenting with STEMI. More than 60 medical centres across North America and Canada contributed data to the registry.

COVID-19 is a proinflammatory, clot forming disease and we now see its effect in the coronary arteries.”Payam Deghani William Wijns
SOLVE-CRT results “offer new hope for patients with hardto-treat heart failure”

Results from a pivotal clinical trial found a leadless pacemaker can deliver cardiac resynchronisation therapy (CRT) among patients who were not able to be treated with conventional CRT and epicardial leads.
The novel WiSE CRT system (EBR Systems) removes the possibility of lead complications and provides physicians with more freedom to target the pacing site within the endocardium of the left ventricle when implanting the device, researchers, presenting the findings during a late-breaking clinical trial session at 2023 Heart Rhythm Society annual meeting (19–21 May, New Orleans, USA) report.
For many patients with heart failure, their two ventricles end up beating at different times in a dyssynchronous rhythm. The current standard of care to help achieve synchrony is CRT, which uses pacing to synchronise the contraction of the right and left ventricles. However, lead-based pacemakers have known limitations and up to 30% of patients fail to benefit from this therapy.
The SOLVE-CRT study is a prospective, international, multicentre pivotal trial that included a randomised part and the subsequent single-arm part. The randomised part of the study was initially intended to enrol 350 patient participants for three indications: patients who were nonresponders (NR), those previously untreated, including acute and chronic lead failures (PU), and patients undergoing high-risk upgrades (HRU)
from existing implanted cardioverter defibrillators (ICD). All participants underwent device implantation and were then randomised in 1:1 ratio to treatment (system turned ON) or control (system turned OFF) groups. A total of 108 participants were enrolled by March 2020 when enrolment was halted due the COVID-19 pandemic.
The single arm part of the study was approved by the US Food and
Drug Administration (FDA) during the COVID-19 pandemic and designed to enrol up to an additional 192 participants within two indications: PU and HRU. An interim analysis was conducted after 75 participants were enrolled. Data from all participants in both parts were included in the interim safety analysis (n=183). The primary safety endpoint for the SOLVE-CRT study was freedom from Type I (device & procedure-related) complications through six months. Efficacy analysis included all participants in the single arm (n=75) and those randomised to treatment from the PU and HRU groups only (n=25), for a total of n=100. The primary efficacy endpoint was the improvement in left ventricular systolic volume (LVESV).
WiSE CRT was shown to be safe with an 80.9% freedom from Type I complications (p<0.001). In addition, there was a significant improvement of 16.4% in left ventricular end systolic
Early anticoagulation after ischaemic stroke “reasonable” and “unlikely to cause harm”
An international clinical trial presented at the European Stroke Organisation Conference (ESOC; 24–26 May, Munich, Germany) has demonstrated that—in people with ischaemic stroke and atrial fibrillation (AF)—anticoagulation can safely be started earlier than current guidelines recommend.
“OUR STUDY FINALLY BRINGS
scientific evidence for a common dilemma in early secondary prevention after an ischaemic stroke,” said study leader Urs Fischer (University Hospital of Bern, Bern, Switzerland), who delivered these findings from the ELAN trial. “In view of our results, early treatment initiation is reasonable if indicated, or if desired, for logistic or other reasons. It is probably better and is unlikely to cause harm.”
Direct oral anticoagulants (DOACs) are used to prevent blood clots in people with AF, but it is unclear how early after stroke they should be started; there are safety concerns surrounding a potential increased risk of bleeding, which may be highest in the first few days post-stroke, but the possible benefit of such drugs may also be highest in these first few days. In the presence of this uncertainty, international
guidelines currently recommend a delay before starting DOACs.
However, as per Fischer’s presentation, the chances of suffering a recurrent event with early DOAC treatment are likely to be lower compared to a later start—without an increase in risk of complications.
The ELAN study included 2,013 participants with an acute ischaemic stroke and AF recruited from 103 different stroke units in 15 countries across Europe, the Middle East and Asia between 2017 and 2022.
Based on the size and location of their infarct on imaging—and, thus, the severity of their stroke— participants were randomly assigned to an early treatment start, or a later, guideline-recommended treatment start. An early start was defined as being within 48 hours of a minor/moderate stroke or day 6–7 following a major stroke, while a late start was
volume (p=0.003). Since both primary endpoints were met at this interim analysis, study enrolment will be concluded early.
“Because heart failure is a progressive disease, patients who fail conventional CRT experience worsening symptoms that can result in repeated hospitalisations and reduced life expectancy. The results of SOLVECRT offer a new hope for patients with hard-to-treat heart failure,” said Jagmeet Singh (Harvard Medical School, Boston, USA), co-principal investigator of the study along with Mary Norine Walsh (Ascension St Vincent Heart Center, Indianapolis, USA). “Endocardial leadless pacing will allow physicians to choose where to implant the device within the left ventricle and the ability to tailor the therapy directly to each individual patient’s needs.”
EBR Systems is working to submit premarket approval (PMA) to the FDA.
defined as day 3–4 following a minor stroke, day 6–7 following a moderate stroke, or day 12–14 following a major stroke. The primary outcome in the ELAN trial was a composite of recurrent ischaemic stroke, symptomatic intracranial haemorrhage (ICH), extracranial bleeding, systemic embolism, or vascular death, within 30 days after randomisation.
At 30 days, the primary outcome occurred in 29 patients (2.9%) in the early treatment group and 41 (4.1%) in the late treatment group. At 90 days, the difference in the rate of the composite outcome was -1.9%. In addition, recurrent ischaemic stroke at 30 days occurred in 14 patients (1.4%) in the early treatment group and 25 (2.5%) in the late treatment group, while—“most importantly”, according to Fischer—symptomatic ICH was similar across the groups, occurring in two patients (0.2%) in both.
“The study also suggests that the incidence of symptomatic intracerebral haemorrhage is low with early anticoagulation, if imaging-based classification is used,” stated Jesse Dawson (University of Glasgow, Glasgow, UK), who led ELAN alongside Fischer.
The findings have been published in the New England Journal of Medicine. Researchers plan to explore whether the risk and benefit is similar in different subgroups of the trial population, especially in stroke patients who are more severely affected.
Endocardial leadless pacing will allow physicians to choose where to implant the device in the left ventricle.”








US FDA removes red flag for use of paclitaxel devices in peripheral interventions
In a letter to healthcare providers dated 11 July 2023, the US Food and Drug Administration (FDA) communicates that the risk of mortality associated with paclitaxelcoated devices to treat peripheral arterial disease (PAD) is no longer supported based on the totality of the available data and analyses.
This update signals a lowering of the red flag raised in a 2019 letter from the Administration— published in response to a meta-analysis that indicated a late mortality signal—warning that treatment of PAD with paclitaxel-coated balloons and paclitaxel-eluting stents was “potentially associated with increased mortality”.
Alongside the letter, the US FDA has updated its recommendations for healthcare providers regarding the use of paclitaxel-coated balloons and stents for PAD. As well as removing reference to the possibility of increased mortality with these devices, the amended guidance softens the language around the monitoring of patients who have been treated with paclitaxel-coated stents and balloons, stating that healthcare providers should continue ‘routine’ rather than ‘close’ monitoring of these patients, as had previously been stated.
The safety of paclitaxel—used in peripheral interventions to prevent restenosis—was called into question by data put forward in 2018 by Konstantinos Katsanos (University of Patras, Patras, Greece) et al that pointed to an increased risk of death at
KEY DATES:
two and five years following the use of paclitaxelcoated balloons and paclitaxel-eluting stents in the femoropopliteal artery.
The FDA responded, notifying healthcare providers in early 2019 about a late mortality signal in patients treated for PAD in the femoropopliteal artery with paclitaxel-coated balloons and paclitaxel-eluting stents. Their most recent update on the topic, prior to that shared on 11 July 2023, was posted in August 2019.
In its new update, the FDA notes that “additional data from the pivotal randomised controlled trials (RCTs) has become available,” and that the
Administration has worked with device manufacturers and external stakeholders to develop the protocol and analysis plan for new data generation.
The FDA references the fact that device manufacturers collaborated in an updated metaanalysis, which included “additional studies, more complete vital status information, and longer-term follow-up compared to prior studies”. Patient followup in these studies ranged from two to five years, the Administration notes, and led it to conclude that the updated RCT meta-analysis “does not indicate that the use of paclitaxel-coated devices is associated with a late mortality signal”.
Furthermore, the FDA states that it also reviewed additional analysed of the risk for late mortality, including the SWEDEPAD trial interim analysis, the VOYAGER PAD study, the German BARMER Health Insurance study, the US Veterans Health Administration study and the Medicare SAFE-PAD study. “None of these studies, with mean or median follow-up ranging from 1.7 to 3.5 years, found a risk for late mortality associated with paclitaxel-coated devices,” the FDA communicates, adding that longerterm follow-up in several of these studies is ongoing.
“Important chapter”
“Today marks an important chapter for the entire vascular community,” commented Eric Secemsky (Beth Israel Deaconess Medical Center, Boston, USA). “The paclitaxel controversy threatened the endovascular space. Patients were unable to access devices demonstrated in randomised trials to improve limb outcomes.
“Physicians were concerned about their patients well being as well as their own legal protection. The reversal by the FDA will now allow us to get these devices to our patients and re-invest in developing novel technology that can serve our patients in the future. We have a lot to learn from this chapter and hopefully we are all better clinicians and scientists because of this experience.”
December 2018
Katsanos metaanalysis finds a higher risk of death in the long term when paclitaxelcoated devices are used in the leg
January 2019
US FDA evaluating paclitaxel data, recommend patient surveillance
“Continued surveillance” is recommended for PAD patients treated with paclitaxel, says the FDA, adding though that the “benefits continue to outweigh the risks” for approved devices used within their indications.
March 2019
US FDA updates letter to healthcare providers
Following preliminary analysis, the FDA recommend that “alternative treatment options to paclitaxelcoated balloons and paclitaxel-eluting stents should generally be used until additional analysis of the safety signal has been performed.”
UK MHRA calls in experts for data review
The UK’s regulatory body, the Medicines and Healthcare products Regulatory Agency, convenes an independent Expert Advisory Group to review the available data.
April 2019
CX audience deems paclitaxel not dangerous
Delegates at the 2019 CX Symposium voted overwhelmingly against the notion that there was a demonstrable danger in any organ of the body attributed to circulating paclitaxel.
June 2019
MHRA limits routine clinical use of paclitaxel devices following expert advisory group advice
The UK’s MHRA acted to limit the future use of paclitaxeleluting stents and paclitaxel-coated balloons in routine clinical care following recommendations from the independent Expert Advisory Group.
FDA panel reviews paclitaxel device data: No recommendations issued, as more work is needed
Following a two-day discussion, an FDA Circulatory System Devices Panel Advisory Committee concluded that there is a “mortality signal” as first described in the Katsanos paper.
August 2019
Paclitaxel device clinical trials “may continue”
The US FDA issues a letter to healthcare providers, in which it states that clinical studies of paclitaxelcoated balloons and paclitaxel-eluting stents “may continue and should collected long-term safety (including mortality) and effectiveness data”.
January 2021
FDA perspective in NEJM highlights need for continued clinical studies on paclitaxel devices
Representatives of the Center for Devices and Radiological Health, US FDA, authored a perspective piece in the New England Journal of Medicine (NEJM), titled, “Drug-coated devices for peripheral arterial disease [PAD]”.
April 2021
CX audience supports call to change recommendations regarding paclitaxel use in peripheral interventions
In the presence of representatives from the US FDA and UK MHRA, an audience vote at CX 2021 saw 74% of participants support that view that “it is time to change the agency recommendations regarding paclitaxel use in peripheral interventions”.
July 2023
US FDA issues letter to healthcare providers updating its position on paclitaxel-coated devices
The US regulator issues a letter to healthcare providers on 11 July, in which it states that, based upon its review of the totality of the available data and analyses, “we have determined that the data does not support an excess mortality risk for paclitaxelcoated devices”.
How the paclitaxel story has developed
The reversal by the FDA will allow us to get these devices to our patients and reinvest in developing novel technology.”
“Not just inventing the next stent”: The future of medtech relies on patient empowerment
Ian Meredith is a well-known figure within the field of interventional cardiology, not only for a distinguished academic and clinical career but also his six-year spell as the executive vice president and global chief medical officer at Boston Scientific.

After retiring from his role at the medtech giant earlier this year, Meredith joined the board of directors at Avertix Medical. The company, formerly known as Angel Medical Systems, is commercialising an implantable cardiac monitor and patientwarning device, the Guardian system, a device designed to detect heart attacks in real time, including silent and atypical symptomatic heart attacks, among patients with acute coronary syndromes.
Meredith spoke to Cardiovascular News to discuss his decision to collaborate with Avertix, how patient interaction is likely to shape the future of cardiovascular medicine, and the current challenges in bringing new technology to the healthcare market.
“What I brought to Boston Scientific was 25 years of caring for patients with acute and chronic ischaemic heart disease, acute and chronic heart failure, and acute and chronic valvular heart disease,” says Meredith of his time at Boston Scientific. “That experience of managing large numbers of patients in the public health system gave me a perspective of a longitudinal approach, rather than a transactional approach to care.” This translated into a strategy looking at developing care that is “parallel to the course of human disease” rather than being “intersectional”, Meredith comments.
Smart diagnostics
After retiring from the post in early 2023, Meredith says that he has sought to engage with companies with whom he can “actively influence the thinking about transforming care to something that is much more longitudinal” and in parallel with patients’ needs. This means, he explains, “not simply inventing the next stent”, but developing smart diagnostics, proper surveillance of the disease process, planning for later stages of disease to minimise rehospitalisation, and delivering care in non-traditional settings, all of which will increase in importance with an ageing global population.
“When the team at Avertix asked if I would be interested, I saw that it fits the bill for the way I think about healthcare in terms of how we provide patients with smart technologies that can prevent hospitalisation, prevent readmission, prevent anxiety and allow people to
live a normal life, and more importantly to empower patients to be able to be engaged and manage their own care,” he adds.
The concept of patients being engaged in their own treatment is one that Meredith sees as being important for the future of the delivery of treatment for cardiovascular disease. “I deeply believe in engaging and empowering patients in their own care. There is an abundance of evidence that patients who are involved in their own care do better and are less anxious about their condition,” he argues. “We have to invert the hierarchy in medicine, empowering patients so that we are there as the facilitators rather than sitting in the ivory tower and passing down dictums from the top.”
This approach is not without its own challenges, not least a potential resistance from healthcare providers, the need for robust evidence underpinning new models, techniques and technologies, regulatory and reimbursement hurdles, as well as the need for an engaged and educated patient population.
“You have to start with meeting the unmet needs with smart, simple, safe,
sophisticated technologies that can provide that sort of surveillance, and the next step, which is a really big one, is providing meaningful evidence, and developing an evidence-generation strategy that actually allows the medical community to have comfort that you are approaching this in a sophisticated and logical way,” comments Meredith.
“And then, of course, the education and training, both of the healthcare provider community and the patient community, so that the system and the solutions are used appropriately.”
Asked how he views the potential for resistance from physicians, particularly those who have been raised in a “top-down” model of care delivery, to engaging patients more closely within the delivery of care, Meredith raises several points.
“To me, the more informed you are, the easier as a patient you are to manage because you understand the subtleties and nuances in the disease process and management outcomes,” he responds. “All of the therapies we have are not perfectly personalised for each individual. They are therapies that are
access to data sources than they would have had in previous decades, a shift that fundamentally changes the way that they need to be treated.
“Everybody does research. Where it is hard is when people are wedded to a doctrine that it is essentially flawed because of their ascertainment bias, when they have collected information reaffirming fixed beliefs. But, I think we live in a world now where people are educating themselves on their health issues, and they are looking for a partner to be shoulder-to-shoulder with them, as opposed to somebody in a patriarchal or hierarchical relationship saying ‘you must do this’.”
Patient-centred care
The Guardian system is one such technology that Meredith sees as playing a role in a more patient-empowered treatment paradigm. The system has US Food and Drug Administration (FDA) class III indication for use in patients who have had prior acute coronary syndrome events and who remain at high risk for recurrence. It works by detecting potential ongoing events,
developed and based upon population evidence, and are largely a good thing for most, but not a perfect fit for many, and having a deep understanding of healthcare makes expectation of outcomes easier.”
With the ubiquity of smartphones and other digital technologies, Meredith says that he believes patients have greater
characterised by sustained ST-segment changes, and alerts the patient to seek medical attention for a potential event.
Meredith says that he expects the accumulation of data as being a core strategy in the continued commercialisation of the device, building upon insights already gathered through previous clinical study, including the ALERTS clinical trial, in which it was shown that patients implanted with the device were able to seek medical attention earlier than patients who relied purely on symptoms.
“Market expansion through thoughtful evidence generation will be the most important next step and then continuing to refine the device,” Meredith comments.
Reflecting on the wider regulatory landscape within the medtech industry, Meredith acknowledges that there have been challenges, particularly in Europe with the introduction of new requirements for medical device manufacturers, but he comments that a focus on patient safety is critical.
“The CE mark system was certainly favourable to industry without necessarily providing the regulatory framework at a level that was ideal,” he says. “You can take it to such extremes that it makes it difficult to develop technology because you simply do not have the financial resources because the regulatory framework is so demanding. There has to be a balance between safe and effective and practical, so that we do not stifle innovation.”
We have to invert the hierarchy in medicine, empowering patients so that we are there as the facilitators rather than sitting in the ivory tower and passing down dictums from the top.”
Product News
procedure easier and safer.
Transfemoral TAVI device gains US FDA breakthrough status for aortic regurgitation
Genesis MedTech has announced that its J-Valve transfemoral (TF) system has been granted breakthrough device designation by the US Food and Drug Administration (FDA).
The valve has been granted breakthrough device designation for the proposed indication of treatment of severe native aortic regurgitation (AR) and AR-dominant mixed aortic valve disease in patients who are judged by a heart team to be eligible for the device and to be at high risk for open surgical aortic valve replacement (SAVR).
The system utilises an anchor mechanism and a stent frame that expands to attach the device to a failing valve.
The J-Valve TF system consists of two components including the J-Valve TF Bioprosthesis and the J-Valve TF delivery device. The bioprosthesis is comprised of bovine pericardium leaflets, a nitinol stent frame covered with polyester fabric and a nitinol anchor ring. The delivery device is used to position the bioprosthesis in the native aortic valve.
“We are hopeful that these designations will aid in providing timely treatment for a condition that currently lacks any transcatheter valves approved in the USA. We look forward to continuing our work with the FDA to bring this life-saving technology to patients in need,” stated Mark A Turco, CEO JC Medical and president of vascular intervention North America at Genesis MedTech.
Robocath’s latest generation robotic PCI platform enters the market
Robocath has announced the launch of its latest robotic platform—the R-One+.
The R-One+ robotic system allows interventional cardiologists to perform percutaneous coronary intervention (PCI) by controlling the devices using an integrated control command unit located in the cath lab or in the control room.
According to Robocath, this creates two main benefits: firstly, the system protects the cardiologist and the medical team against radiation-induced injuries, and secondly it makes the PCI
“For the first time, I was able to perform several robotic angioplasties from the comfort of my chair in the control room, where I was completely shielded from X-rays and could dispense with my lead apron,” said Mohammed Nejjari (Centre Cardiologique du Nord, Saint-Denis, France). “I also benefited from the excellent visibility on the radioscopy and haemodynamic monitoring screens. This new set-up has changed the way we organise our procedures and has given nursing staff the opportunity to develop new skills.
“This new approach has also enabled doctors to shift their focus back to their core activities and to perform procedures in complete safety and with millimeter precision. I am excited to incorporate this new robotic technology into my day-to-day work. Without doubt, it opens up some new and extremely promising avenues for the treatment of cardiovascular diseases, especially in the case of long and complex procedures.”
(University College London Hospitals, London, UK). “Whether on the ward or in general practice, being able to provide high-quality imaging at the point of care means rapid diagnosis and rapid treatment.”
The device is currently commercially available in select countries throughout Europe and Asia as well as Australia and New Zealand, while in the USA, Vscan Air SL is 510(k) cleared by the US Food and Drug Administration (FDA) and is expected to become commercially available this quarter.

generation Shockwave C2 device, and is optimally designed to treat longer calcified lesions and more challenging eccentric and nodular calcium, the company said in a press release.
GE Healthcare launches point-of-care ultrasound system Vscan Air SL
GE Healthcare has announced the launch of Vscan Air SL, a dual-headed, handheld, wireless ultrasound imaging system designed for rapid cardiac and vascular assessments at the point of care.
The device is the latest-generation in GE Healthcare’s Vscan family of products, and features GE Healthcare’s proprietary SignalMax and XDclear technology.
The company describes the Vscan Air SL as a “pocket-sized, portable tool that allows for clear, wholebody scanning and secure viewing of images”. Through Vscan Air + Digital Tools, clinicians will have access to subscriptions that can connect them to a suite of solutions designed to improve workflow with secure collaboration, image, and device management features.
The Vscan Air app, available on Android and iOS devices, allows for the secure viewing of images on a mobile device so clinicians can take the device with them to every patient.
“Having ever more powerful handheld ultrasound is a game changer for patient care,” said Guy Lloyd

Pacing
wire for TAVI and balloon aortic valvuloplasty procedures gains US FDA clearance
Teleflex has received US Food and Drug Administration (FDA) clearance for the Wattson temporary pacing guidewire.
The approval expands the company’s structural heart portfolio with the first commercially available bipolar temporary pacing guidewire designed specifically for use during transcatheter aortic valve implantation (TAVI) and balloon aortic valvuloplasty (BAV).
In a press release, Teleflex states that the device features a simple design to create procedural efficiencies, as well as offering dual functionality, by supporting both valve delivery and ventricular bipolar pacing during TAVI or BAV procedures.

“This technology enables us to provide physicians with a new tool specifically engineered to address unmet clinical needs frequently encountered during TAVI or BAV procedures,” said Jake Newman, president of the Americas at Teleflex. “The Wattson temporary pacing guidewire reflects our focus on purposeful innovation and commitment to providing more options to further simplify minimalist TAVI and other structural procedures.”
The device will enter a limited market release phase, with full market release anticipated later this year.
“Shockwave C2+ maintains the intuitive catheter design and ease of use that are foundational to the success of Shockwave IVL and incorporates improvements that will enhance procedural efficiency and optimise the treatment of the most challenging morphologies,” said Jonathan Hill (Royal Brompton Hospital, London, UK). “The extra pulses are most advantageous in areas with the highest burden of calcium, including nodular, eccentric, diffuse and multivessel calcium.”
Shockwave C2+ is commercially available for the treatment of de novo coronary artery disease in Europe and select other geographies.
Siemens unveils Acuson Origin imaging platform
Siemens Healthineers unveiled the Acuson Origin, a dedicated cardiovascular ultrasound system with artificial intelligence (AI) features, at the European Society of Cardiology (ESC) congress (25–28 August, Amsterdam, The Netherlands). The system is designed to help physicians perform minimally invasive cardiac procedures more efficiently, and addresses the entire continuum of cardiovascular patient care, including diagnostic, structural heart, electrophysiological, and paediatric procedures.
Acuson Origin includes a suite of AI-powered features for measurements, view recognition, and imaging assistance, trained on a large cardiac image database and intended to save the user time on diagnostic echocardiography procedures by providing automated measurements that are accurate and reproducible. More than 500 AI-powered measurements on transthoracic and transesophageal echocardiography are designed to enhance cardiovascular workflows and reduce variability.
The system also delivers automated contouring and quantification of all four cardiac chambers without the need for an electrocardiogram (ECG) via the 2D and 4D HeartAI features. Leveraging the latest advancements in AI, 2D HeartAI opens up auto tracking on contrast images.
Shockwave Medical’s C2+ coronary IVL catheter launched in select markets
Shockwave Medical has announced the full commercial availability of the Shockwave C2+ coronary intravascular lithotripsy (IVL) catheter to treat severely calcified coronary artery disease in select international markets. Shockwave C2+ provides 50% more pulses per catheter than the previous-
4D HeartAI is a dual modality advancement for 4D auto contouring of all four chambers in 4D transthoracic echocardiography (TTE) and both ventricles in 4D transesopageal echocardiography (TEE), averaging up to five beats with or without ECG. The system is the first to provide real-time cardiac view recognition. AI Assist, a new, AI-driven feature specific to TTE, recognises cardiac structures the moment the transducer makes contact with the chest wall. The implementation of this cardiac view classification feature is Colour and Spectral Doppler placement.
Clinical News
Harmonization By Doing programme, jointly established by the FDA and Japan’s Pharmaceutical and Medical Device Agency (PMDA), the clinical trial will be conducted concurrently at 10 centres in the USA and five centres in Japan, with 60 patients expected to be enrolled.
First two US patients receive Trisol tricuspid valve
The heart teams at the Piedmont Heart Institute (Atlanta, USA) and the University of Virginia Health System (Charlottesville, USA) successfully performed the first two implantations in the USA of the Trisol transcatheter tricuspid valve replacement system (Trisol Medical) as part of a US Food and Drug Administration (FDA) approved early feasibility study (EFS), led by principal investigator Isaac George (Columbia University, New York, USA).
Shimon Eckhouse, Trisol’s chairman, said: “There is a huge unmet need for a transcatheter solution to treat severe tricuspid regurgitation (TR). Trisol’s promising initial clinical data instills confidence that Trisol can play a major role in this domain.”
The patented valve is comprised of a single leaflet, affixed by two commissures enabling it to function as a bileaflet valve. According to Trisol Medical, this design facilitates a slower closing of the leaflets, a feature that is intended to preserve the right ventricular function following the valve replacement. Trisol’s valve employs axial anchoring, that reduces the risk of conductive issues.
To date, the Trisol Valve has been implanted in ten people. Five of these implants were performed as part of the Israeli pilot study, led by principal investigator Ran Kornowski (Rabin Medical Center, Tel Aviv, Israel). Currently, the longest follow-up period exceeds two years. Trisol aims to complete its EFS and initiate pivotal studies in 2024.
VenusP-Valve gains FDA authorisation for transpacific clinical study
Venus Medtech has announced US Food and Drug Administration (FDA) investigational device exemption (IDE) approval for its VenusP-Valve percutaneous pulmonary valve system.
The device becomes the first Chinese-developed heart valve system to be approved by the FDA for a clinical trial, “setting a new milestone in the global footprint of Chinese valves,” the company says in a press release.
The IDE approval will allow VenusPValve to initiate pivotal clinical trials in the USA to support premarket approval (PMA). Through the Japan-US
The device is set to enrol patients in the USA in the second half of this year and in Japan in early 2024, with approvals expected around 2026 in both markets. VenusP-Valve has been used in two compassionate-use cases in the USA, and received the CE mark under the European Medical Devices Regulation (MDR) in April 2022.
registration application to the National Medical Products Administration (NMPA).
Currently, there are no transfemoral TAVI systems approved by the NMPA in China for the AR indication. In June 2023, TaurusTrio TAVI system was accepted by the Special Review and Approval Procedure for innovative medical devices of the NMPA.
INFINITY-SWEDEHEART
trial of DynamX bioadaptor system completes enrolment
Elixir Medical has announced the completion of enrolment in INFINITYSWEDEHEART, a prospective, multicentre, single-blind, randomised clinical trial of the DynamX coronary bioadaptor system, a metallic coronary artery implant that adapts to vessel physiology. The trial will see the device compared against the Resolute Onyx (Medtronic) drug-eluting stent (DES).
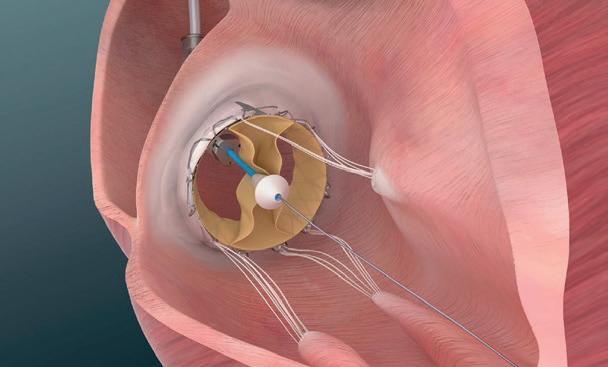
TaurusTrio TAVI device gets first implants in aortic regurgitation clinical study
Peijia Medical has announced that the first Chinese patient has been enrolled in the clinical trial of its TaurusTrio transcatheter aortic valve implantation (TAVI) device
TaurusTrio is the licensed version of JenaValve’s Trilogy heart valve system, which has been developed for the treatment of aortic regurgitation (AR), after Peijia Medical obtained an exclusive licence for the development, manufacture and commercialisation of the device in the Greater China region in January 2022.
The first implant was successfully completed by Yongjian Wu and Guangyuan Song at Beijing An Zhen Hospital, Capital Medical University on 26 July.

“We are pleased to have safely implanted the first patient in China with the TaurusTrio TAVI system,” said Yi Zhang, chief executive officer of Peijia Medical. “The trial reinforces our goals of improving care in the field of cardiology and expanding TAVI systems within China. We are looking forward to our continued partnership with American company, JenaValve, and presenting further data from the clinical trial at a future date.”
The clinical trial of the TaurusTrio TAVI system is designed to assess the safety and efficacy of the system for treating patients with native symptomatic, severe AR, who are judged by a heart team to be at high or greater risk for surgical aortic valve replacement (SAVR). The result of the clinical trial would be included in the company’s future submission of
The trial includes 2,400 patients from 14 sites across Sweden. The primary device-oriented clinical endpoint is target lesion failure (TLF); a composite of cardiovascular death, target vessel myocardial infarction (TV-MI), and ischaemia-driven target lesion revascularisation (ID-TLR). Secondary endpoints include testing for superiority in reduction of TLF and angina pectoris with the bioadaptor over DES in all patients and in prespecified subgroups.
“For more than 20 years, generations of drug-eluting stents have offered no hard clinical benefits beyond the first year for the treatment of ischaemic heart disease and no advancements have been made to address the annual non-plateauing adverse events,” said David Erlinge (Lund University, Lund, Sweden), principal investigator of the INFINITY-SWEDEHEART RCT.
“This trial is conducted through a robust national Swedeheart registry and includes patients we typically see in our practices, with both chronic and acute coronary syndromes, and will provide important data to potentially change the future of PCI treatment,” commented Stefan James (Uppsala University Hospital, Uppsala, Sweden), chairman of the Swedeheart Steering Committee.
INFINITY-SWEDEHEART is the fourth trial of Elixir Medical’s DynamX bioadaptor clinical evidence programme consisting of nine company-sponsored and investigatorinitiated studies enrolling more than 9,000 patients.
The DynamX Coronary Bioadaptor System is CE marked. The device is not available for sale in the USA.
First patients treated in Indian clinical trial of Tria surgical mitral valve
Foldax has announced that the first 30 patients have been treated outside of the USA in the Indian clinical trial of the Tria surgical mitral heart valve.
The Tria mitral valve is designed to accommodate the anatomy and pressures of the mitral position. The device combines the company’s
proprietary polymer—LifePolymer— with a valve design intended to resist calcification, withstand stresses and strains without failure, and restore patient quality of life without lifelong use of anticoagulants.
The Indian trial is a prospective surgical mitral valve study that will enrol up to 70 adult patients with mitral valve disease from 10 sites and will support commercialisation in India. Valve haemodynamic and durability performance will be evaluated at six months and one year.The Tria heart valve is for investigational use only and is not available for commercial sale.
Enrolment begins in LOVEDEB study of Selution SLR sirolimus-eluting balloon
The first patient has been enrolled in a UK study of large vessel de novo coronary artery disease treated with Selution SLR (MedAlliance) sirolimuseluting balloon.This is a trial initiated by physicians at the Wrightington, Wigan and Leigh Teaching Hospitals NHS Foundation Trust, where the first patient was enrolled.
The aim of the LOVE-DEB Study (Large de-novo coronary artery disease treated with sirolimus drug-eluting balloon: prospective evaluation of safety & efficacy of Selution SLR drug-eluting balloon) is to evaluate the safety and efficacy of Selution SLR in treating native de novo coronary artery disease in larger vessels (≥2.75mm). Its primary objective is to evaluate the proportion of patients who underwent target lesion revascularisation (TLR) within one year of their procedure.
“The LOVE-DEB study is a unique trial in which we are assessing the safety and efficacy of sirolimus DEB in de novo disease only in the large coronary arteries in routine clinical practice,” said principal investigator, Abhishek Kumar (Wrightington, Wigan and Leigh Teaching Hospitals NHS Foundation Trust, Wigan, UK). “We aim to recruit 300 patients over a period of 12 months across 10 centres in the UK. I am extremely delighted that we have kick-started the study by recruiting our first patient in the study at Wigan. I am very grateful for the invaluable support from all those who are involved in this study.”
Currently three investigational device exemption (IDE) clinical studies are evaluating Selution SLR in the USA: in chronic limb-threatening ischaemia (CLTI) patients with belowthe-knee (BTK) disease; superficial femoral artery (SFA) and proximal popliteal artery (PPA); and coronary in-stent restenosis (ISR).
MedAlliance received IDE approval from the US Food and Drug Administration (FDA) for de novo coronary artery lesions in January 2023. Selution SLR was awarded CE mark approval for the treatment of coronary artery disease in May 2020. This complements the experience that the company has gained with the SELUTION DeNovo and SUCCESS trials in Europe.
Industry News
Neurointerventional Surgery, and ISNR Stroke
“I have been impressed by the Protembis team’s achievements in developing an elegant system to mitigate cerebral infarction risk during TAVI,” Mocco is quoted as saying in a press release. “Their adaptive IDE clinical trial strategy is both rigorous and innovative. I am excited to offer my insights and guidance to the board as this field evolves to treat future aortic stenosis patients who will have zero tolerance for brain injury as a potential procedural complication”.
Stroke expert J Mocco joins Protembis board
Protembis has announced the appointment of J Mocco (Icahn School of Medicine at Mount Sinai, New York, USA) as an independent member of its board of directors.

Mocco will offer insights into the strategic direction of the company as well as guidance on clinical strategies and new product development, the company said in a press release, as it seeks to bring its ProtEmbo cerebral protection system to market.
Protembis has recently received US Food and Drug Administration (FDA) approval to conduct an investigational device exemption (IDE) study aimed at demonstrating safety and efficacy of the ProtEmbo system during transcatheter aortic valve implantation (TAVI). The IDE study is designed as a multicentre randomised controlled trial in the USA and Europe.
Mocco brings a wealth of clinical and academic experience as the Kalmon D Post professor and senior vice chair of the Department of Neurological Surgery at Mount Sinai and is the immediate past president of the Society of Neurointerventional Surgery (SNIS). In a career spanning more than 20 years, he has authorship credits on over 600 publications, and is an editorial board member of Stroke since 2015 as well as serving as an associate editor of other journals including Neurosurgery, the Journal of
Conference calendar
2023
4–7 October
European Association for Cardio-Thoracic Surgery (EACTS) Annual Meeting Vienna, Austria eacts.org/annual-meeting
23–26 October
TCT 2023 San Francisco, USA tct2023.crfconnect.com
Radiaction Medical secures Series C2 funding and announces board addition
Radiaction Medical has raised US$12.6 million in Series C2 funding led by US private equity fund InnovaHealth Partners with participation from additional co-investors and has announced the election of Christopher Barys to serve on its board of directors.
Barys joins the organisation with more than 30 years of medical device sales, marketing, and business experience, including leading growth and innovation within the cardiac catheterisation and imageguided spaces.
Barys said: “I am thrilled to be a part of Radiaction’s mission to provide advanced radiation protection to the entire procedural staff. Now more than ever, the medical community needs innovative solutions to pressing systemic problems. Radiaction is disrupting the industry for the better. I am looking forward to supporting Radiaction in bringing its radiation reduction solution to every catheterisation lab in the USA and abroad.”
The Radiaction system is designed to proactively block radiation at the source, providing head-to-toe protection to the entire interventional team while allowing unencumbered patient care, access, and service, the company says in a press release.
7–10 November
Cardiovascular Interventions La Jolla, USA cvinterventions.com
11–13 November
American Heart Association (AHA) 2023 Scientific Sessions Philadelphia, USA professional.heart.org
19–21 November PCR London Valves 2023 London, UK pcronline.com/courses
Jonathan Yifat, Radiaction CEO, adds: “This current financing round is a significant step forward, one that enables us to expand our reach further, lead with exceptional customer support, and provide this essential technology to protect those who care for patients.
“The addition of Christopher Barys is a tremendous asset to the strategic composition of our board of directors. Chris has a proven track record of market development and growth within the interventional space we aim to serve. He brings a keen eye for strategic opportunities and will help guide our company through its next stages of growth.”
Laplace Interventional secures funding for clinical testing of transcatheter tricuspid valve technology
Laplace Interventional has completed its first close on its Series B financing, co-led by ShangBay Capital along with Features Capital, including participation from Engage Venture Partners, JWC Ventures, as well as a global strategic investor.
The US$12.9M Series B financing will fund Laplace Interventional towards achieving its early clinical feasibility milestones. The company also announced that Jenny Barba, co-founder and managing partner at Features Capital, joined its board of directors and Todd Mortier, a medtech entrepreneur, had previously joined its board of directors.
Laplace Interventional’s transcatheter device is designed to offer an increase in life expectancy and an improvement to the quality of life to patients diagnosed with tricuspid regurgitation (TR). The company is developing a prosthetic valve that will be delivered through patient vasculature not requiring an open-heart surgery and thereby reducing complications in patients.
“Our team at Laplace has demonstrated feasibility of our technology in a preclinical setting and we are well positioned to execute our clinical feasibility evaluation in the near future,” said Ramji Iyer, founder and CEO of Laplace Interventional. “We are grateful for the validation of our progress from our new and existing
2024 27–29 January Society of Thoracic Surgery (STS) Annual Meeting San Antonio, USA sts.org/meetings
9–12 March Cardiovascular Research Technologies (CRT) 2024 Washington, DC, USA crtmeeting.org
investors. We remain laser focused on our mission of improving the standard of care for patients suffering from tricuspid regurgitation.”
Laplace Interventional’s device is in the early stage of development phase and is not approved or cleared by the US Food and Drug Administration (FDA) or any other regulatory body in any region of the world.
Michael Jaff joins board of directors at HeartBeam
HeartBeam, developer of a 3D-vector electrocardiogram (VECG) platform, has announced the appointment of Michael Jaff to its board of directors. Jaff is chief medical officer and vice president of clinical affairs, technology and innovation of the Peripheral Interventions division at Boston Scientific. Previously, he was a professor of medicine at Harvard Medical School (Boston, USA) and he served as president of NewtonWellesley Hospital (Newton, USA).
In a press release, HeartBeam described Jaff as a recognised expert in all aspects of vascular medicine. Jaff is the founder of VasCore, the Vascular Ultrasound Core Laboratory and has published over 300 peer-reviewed publications and 10 textbooks.

“I am passionate about innovation in healthcare and am excited to join the board at HeartBeam,” said Jaff. “Their technology has the potential to bring standard of care cardiac diagnostic technology directly to patients, improving outcomes and saving lives while reducing unnecessary expenditures. I look forward to contributing to these efforts.”
14 March Global Cardiovascular Awards London, UK globalcardiovascularawards.com
21–23 March
JIM GISE Congress Milan, Italy jimgise2024.it
6–8 April
American College of Cardiology (ACC) scientific session
Atlanta, USA expo.acc.org
23–25 April
Charing Cross (CX) Symposium London, UK cxsymposium.com
Onyx Frontier™ Zotarolimus-Eluting Coronary Stent System Brief Statement

Indications
The Onyx Frontier™ zotarolimus-eluting coronary stent system is indicated for improving coronary luminal diameters in patients, including those with diabetes mellitus or high bleeding risk, with symptomatic ischemic heart disease due to de novo lesions of length ≤ 35 mm in native coronary arteries with reference vessel diameters of 2.0 mm to 5.0 mm. In addition, the Onyx Frontier™ zotarolimus-eluting coronary stent system is indicated for treating de novo chronic total occlusions and non-left main bifurcation lesions utilizing the provisional bifurcation stenting technique.
Contraindications

The Onyx Frontier system is contraindicated for use in: • Patients with a known hypersensitivity or allergies to aspirin, heparin, bivalirudin, clopidogrel, prasugrel, ticagrelor, ticlopidine, drugs such as zotarolimus, tacrolimus, sirolimus, everolimus, or similar drugs or any other analogue or derivative • Patients with a known hypersensitivity to the cobalt-based alloy (cobalt, nickel, chromium, and molybdenum) or platinum-iridium alloy • Patients with a known hypersensitivity to the BioLinx™ polymer or its individual components. Coronary artery stenting is contraindicated for use in: • Patients in whom antiplatelet and/or anticoagulation therapy is contraindicated • Patients who are judged to have a lesion that prevents complete inflation of an angioplasty balloon or proper placement of the stent or stent delivery system.
Warnings
• Ensure that the inner package has not been opened or damaged as this would indicate the sterile barrier has been breached. • The use of this product carries the same risks associated with coronary artery stent implantation procedures, which include subacute and late vessel thrombosis, vascular complications, and bleeding events. • This product should not be used in patients who are not likely to comply with the recommended antiplatelet therapy.
Precautions
• Only physicians who have received adequate training should perform implantation of the stent.


• Subsequent stent restenosis or occlusion may require repeat catheter-based treatments (including balloon dilatation) of the arterial segment containing the stent. The long-term outcome following repeat catheterbased treatments of previously implanted stents is not well characterized. • The risks and benefits of the stent implantation should be assessed for patients with a history of severe reaction to contrast agents.
• Do not expose or wipe the product with organic solvents such as alcohol. • The use of a drug-eluting stent (DES) outside of the labeled indications, including use in patients with more tortuous anatomy, may have an increased risk of adverse events, including stent thrombosis, stent embolization, myocardial infarction (MI), or death. • Care should be taken to control the position of the guide catheter tip during stent delivery, stent deployment, and balloon withdrawal. Before withdrawing the stent delivery system, confirm complete balloon deflation using fluoroscopy to avoid arterial damage caused by guiding catheter movement into the vessel.
• Stent thrombosis is a low-frequency event that is frequently associated with MI or death. Data from the RESOLUTE clinical trials have been prospectively evaluated and adjudicated using the definition developed by the Academic Research Consortium (ARC).




The safety and effectiveness of the stent have not yet been established in the following patient populations:
risks and benefits of the procedure should be weighed against the possible risk associated with interruption of antiplatelet therapy. Patients who require premature DAPT discontinuation should be carefully monitored for cardiac events. At the discretion of the patient’s treating physician(s), the antiplatelet therapy should be restarted as soon as possible.
Instructions for Stenting of Bifurcation Lesions
The provisional technique of bifurcation stenting recommends a single stent placement in the Main Vessel (MV), finalized with proximal optimization technique (POT). POT includes performing post-dilatation to achieve full apposition of the stent proximal to the bifurcation and reduce the risk of side branch (SB) compromise. If inadequate results are found in the SB such as: threatened SB closure, TIMI flow < 3, dissection type B or worse, or residual stenosis > 80%, the provisional bifurcation stenting technique recommends placing a second stent in the SB as a bailout. As per cardiology societal recommendations, two-stent techniques following single stent provisional bifurcation stenting including T, TAP, and Culotte stenting may be utilized as needed. However, the RESOLUTE ONYX PAS Bifurcation Cohort did not evaluate the safety and effectiveness of two-stent bifurcation techniques, including planned (upfront) two-stent bifurcation techniques (such as DK-crush). Additionally, two-stent bifurcation techniques may introduce additional forces and/or failure modes to the stents, and the performance of the Resolute Onyx stent has not been evaluated under these conditions in nonclinical testing.
Potential Adverse Events


Other risks associated with using this device are those associated with percutaneous coronary diagnostic (including angiography and IVUS) and treatment procedures. These risks (in alphabetical order) may include but are not limited to: • Abrupt vessel closure • Access site pain, hematoma, or hemorrhage • Allergic reaction (to contrast, antiplatelet therapy, stent material, or drug and polymer coating) • Aneurysm, pseudoaneurysm, or arteriovenous fistula (AVF) • Arrhythmias, including ventricular fibrillation • Balloon rupture • Bleeding • Cardiac tamponade • Coronary artery occlusion, perforation, rupture, or dissection • Coronary artery spasm
• Death • Embolism (air, tissue, device, or thrombus) • Emergency surgery: peripheral vascular or coronary bypass • Failure to deliver the stent • Hemorrhage requiring transfusion • Hypotension/hypertension
• Incomplete stent apposition • Infection or fever • MI • Pericarditis • Peripheral ischemia/peripheral nerve injury • Renal failure • Restenosis of the stented artery • Shock/pulmonary edema • Stable or unstable angina • Stent deformation, collapse, or fracture • Stent migration or embolization • Stent misplacement • Stroke/ transient ischemic attack • Thrombosis (acute, subacute, or late)
Adverse Events Related to Zotarolimus
Patients’ exposure to zotarolimus is directly related to the total amount of stent length implanted. The actual side effects/complications that may be associated with the use of zotarolimus are not fully known. The adverse events that have been associated with the intravenous injection of zotarolimus in humans include but are not limited to: • Anemia • Diarrhea • Dry skin • Headache • Hematuria • Infection • Injection site reaction
• Pain (abdominal, arthralgia, injection site) • Rash
The potential adverse reactions in nursing infants from zotarolimus have not been determined. The pharmacokinetic and safety profiles of zotarolimus in infants are not known.
Adverse Events Related to BioLinx polymer



• Patients with target lesions that were treated with prior brachytherapy or the use of brachytherapy to treat in-stent restenosis of the stent
• Women who are pregnant or lactating
• Men intending to father children
Although the type of risks of the BioLinx™ polymer coating are expected to be no different than those of other stent coatings, the potential for these risks are currently unknown as the coating has limited previous use in humans. These risks may include but are not limited to the following: • Allergic reaction


• Pediatric patients below the age of 18 years
• Patients with coronary artery reference vessel diameters of < 2.0 mm or > 5.0 mm
• Patients with evidence of an acute ST-elevation MI within 72 hours of intended stent implantation
• Focal inflammation at the site of stent implantation • Restenosis of the stented artery
• Patients with vessel thrombus at the lesion site
• Patients with diffuse disease or poor flow distal to identified lesions
• Patients with lesions located in a saphenous vein graft, in the left main coronary artery, or ostial lesions
• Patients with 3 vessel disease
The safety and effectiveness of the stent have not been established in the cerebral, carotid, or peripheral vasculature. Additionally, the safety and effectiveness of using atherectomy devices with the stent have not been established. The effect of potential drug interactions on the safety or effectiveness of the Onyx Frontier stent has not been investigated. Potential interactions of the stent with other drug-eluting or coated stents have not been evaluated and should be avoided whenever possible.
Clinical studies of the Resolute stent did not suggest any significant differences in safety and effectiveness for male and female patients and did not include sufficient numbers of patients to assess for differences in safety and effectiveness due to ethnicity.
Decisions about duration of DAPT are best made on an individual basis and should integrate clinical judgment, assessment of the benefit/risk ratio, and patient preference. Premature discontinuation or interruption of prescribed antiplatelet medication could result in a higher risk of stent thrombosis, MI, or death. Before PCI, if premature discontinuation of antiplatelet therapy is anticipated, physicians should carefully evaluate with the patient whether a DES and its associated recommended DAPT regimen is the appropriate PCI choice.
Following PCI, if elective noncardiac surgery requiring suspension of antiplatelet therapy is considered, the
Please reference appropriate product Instructions for Use for more information regarding indications, contraindications, warnings, precautions, and potential adverse events.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For further information, please call and/or consult Medtronic at the toll-free numbers or websites listed. Medtronic
Onyx Frontier™ DES
Built for bifurcation
Onyx Frontier DES is indicated for use in non-LM bifurcations† with a unique single-wire design to optimize bifurcation PCI, helping you:
Treat complex bifurcation anatomy with a highly conformable stent platform1

Access the side branch more easily with rounded struts2
Open the stent cell to the side branch while maintaining consistent scaffolding3 and strength4
†Onyx Frontier DES is indicated for use in non-LM bifurcations using the provisional technique.
Visit our website to see how Onyx Frontier DES is built for bifurcation.†
medtronic.com/OnyxFrontier
